Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yasushi Shintani and Version 3 by Catherine Yang.
In the tumor microenvironment, cancer-associated fibroblasts (CAFs) have multiple tumor-promoting functions in drug resistance, regulation of the niche of cancer stem cells and formation of the immunosuppressive network. Lung cancer is the most frequently diagnosed cancer and the leading cause of cancer death worldwide. The most common lung cancer is non-small cell lung cancer (NSCLC), with an overall 5-year survival rate of around 20% because NSCLC is a metastatic disease. CAFs interact with lung cancer cells to allow for the acquisition of malignancy and treatment resistance by paracrine loops via EMT signals in the tumor microenvironment
- non-small cell lung cancer
- tumor microenvironment
- cancer-associated fibroblasts
1. Signaling Pathways between CAFs and NSCLC
Cancer initiates fibroblast transition with acquisition of CAF phenotypes via cancer-derived growth factors, as well as cytokines that regulate the TGF-β and NF-κB signaling pathways [1]. We have previously shown that TGF-β secreted from NSCLC cancer cells activates fibroblasts in the tumor microenvironment [2]. CAFs secrete growth factors, including TGF-β, FGF2/7, PDGF, and HGF, as well as VEGF, which promote cancer cell proliferation [3]. Resident fibroblasts are also activated and change to proinflammatory CAFs during the early preneoplastic stages of tumorigenesis [4]. Activated CAFs produce higher amounts of SDF-1 than lung normal fibroblasts (LNFs), and SDF-1 facilitates cancer cell proliferation and chemoresistance via the CXCR4-mediated signaling pathway, which involves NF-κB and Bcl-xL [5]. MAPK, PI3K/mTOR, and Wnt/β-catenin signaling are also activated in cancer cells in response to CAF-derived growth factors and cytokines. The JAK/STAT pathway are activated by CAF-derived IL-6, IL-10, IL-11, and IL-22 [6]. We previously reported that NSCLC cells underwent EMT and acquired stem-cell-like properties when cocultured with CAFs isolated from surgical exploration [7]. IL-6 from CAFs enhanced EMT and chemoresistance in NSCLC cells, suggesting a role of IL-6 in the maintenance of a paracrine loop that functions as part of the communication between CAFs and NSCLC cells [2][8]. Similarly, CAF-derived CXCL1, IL-6, and COX-2, known targets of the NF-κB transcription factor, reportedly correlate with tumor-promoting inflammation and tumor invasiveness [9][10]. Iwai et al. reported altered gene expression in NSCLC CAFs compared to LNFs using capped analysis of gene expression to comprehensively analyze promoter activity in CAFs [11]. Among 390 genes highly expressed in NSCLC CAFs, they identified COLXIα1, integrin α11, and COL1A1 as CAF-specific genes that promote CAFs migration toward collagen type I and fibronectin via ERK1/2. Thus, several different signaling pathways in CAF-mediated cancer progression have been extensively explored to determine their roles in the tumor microenvironment (Figure 1). All these signaling pathways in CAFs have potential as targets for blocking crosstalk between CAFs and cancer cells.
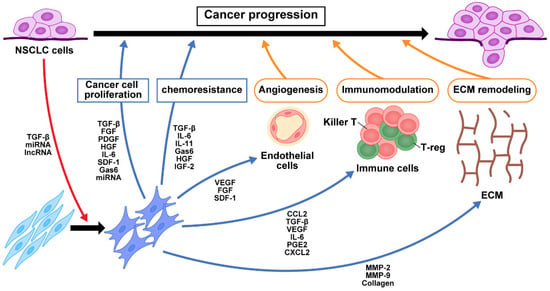
Figure 1. Crosstalk of signaling pathways among CAFs, NSCLC cells, and immune cells. TGF-β and exosomes secreted from NSCLC cancer cells activate fibroblasts into CAFs in the tumor microenvironment. The production of growth factors, cytokines, chemokines, and exosomes from CAFs contribute to NSCLC cell proliferation, chemoresistance, angiogenesis, immunomodulation, and ECM remodeling. These in turn change the tumor microenvironment and contribute to NSCLC progression. NFs, normal fibroblasts; CAFs, cancer-associated fibroblasts; NSCLC, non-small cell lung cancer; miRNA, microRNA; lncRNA, long non-coding RNA; TGF-β, transforming growth factor-β; FGF, fibroblast growth factor; PDGF, platelet-derived growth factor; HGF, hepatocyte growth factor; IL-6, interleukin-6; SDF-1, stromal cell-derived factor-1; Gas6, growth arrest-specific 6; IL-11, interleukin-11; IGF2, insulin-like growth factor 2; VEGF, vascular endothelial growth factor; CCL2, C-C motif chemokine 2; PGE2, prostaglandin E2; CXCL2, chemokine (C-X-C motif) ligand 2; MMP, matrix metalloproteinase; ECM, extracellular matrix.
2. Role of CAFs in Resistance to Antitumor Therapy
EMT resulted in increased malignant potential and reduced sensitivity to chemotherapy in NSCLC cells [12]. Furthermore, chronic exposure to anticancer drugs or radiation resulted in cells forming therapy-resistant sublines that underwent EMT via interactions between NSCLC cells and CAFs through the TGF-β, and IL-6 pathways [2]. Exposure to anticancer drugs enhances IL-11 secretion by CAFs, which promotes chemoresistance of cancer cells through the STAT3 signaling pathway [10][13]. CAF expression of Gas6, a natural ligand of tumor-associated macrophage (TAM) receptors with high affinity for the receptor tyrosine kinase Axl, increases during chemotherapy and promotes proliferation and migration of NSCLC cells [14]. Tumoral Axl activation induces EMT and promotes cell survival and chemoresistance [15]. In addition, CAFs enhanced chemoresistance by repression of caspase-3 and caspase-8 through the activation of the annexin A3/JNK pathway [16]. CAFs also induce acquired chemoresistance through the IGF2/IGFR-1 paracrine pathway, which activates IGF2/AKT/Sox2/ABCB1 signaling and upregulation of P-glycoprotein expression in NSCLC cells [17]. Such data suggest that CAFs, in concert with tumor cells and other components of the tumor microenvironment, abet resistance to treatment [18].
3. Role of CAFs in Oncogene Addicted NSCLC
Numerous driver mutations have been identified in NSCLC, and oncogene addiction provides rationale for molecular targeted therapy [19]. Pellinen et al. recently explored associations of EGFR mutations and CAF subtypes using multiplex fluorescence immunohistochemistry; their findings indicated that gene alterations may affect the properties of CAFs in the tumor microenvironment [20]. That microenvironment provides sustained resistance to molecular targeted therapy. CAFs have a critical role in the resistance of lung cancer to EGFR TKIs through the induction of EMT via CAF-mediated signaling pathways [21][22]. Yoshida et al. also found that lung adenocarcinoma cell lines became more resistant to EGFR TKIs when cocultured with CAFs expressing podoplanin, suggesting that podoplanin-positive CAFs may be useful for predicting response to EGFR TKIs [23]. CAF-released IL-6 mediates NSCLC acquired resistance to EGFR TKIs through the JAK1/STAT3 pathway [24]. In addition, MET activation is an important mechanism for acquisition of resistance to TKIs [25]. Treatment of EGFR- or MET-addicted NSCLC cancer cells was shown to cause a metabolic shift toward increased glycolysis and lactate production, which develop chemoresistance in NSCLC cells [26]. Another study demonstrated that CAFs increase the expression and phosphorylation of annexin A2 by secretion of HGF and IGF-1, which regulate EMT and EGFR TKI resistance in a paracrine manner [22]. Together, these findings suggest that cotreatment targeting CAFs may further improve the antitumor efficacy of molecular targeted therapy.
4. Role of Extracellular Vesicles in Communication between CAFs and NSCLC
Exosomes carry and transfer a variety of cargo, including small non-coding RNAs, also known as miRNAs and lncRNAs, which have essential roles in cellular communication. Shen et al. compared miRNA expression profiles between CAFs from NSCLC and LNFs from matched healthy lungs and showed downregulation of miR-1 and miR-206 and upregulation of miR-31 in CAFs [27]. CAF-derived miRNAs also contribute to the transformation of LNFs into CAFs [28]. Furthermore, miR-210 in the exosome secreted by CAFs was taken up by NSCLC cells and promoted EMT by targeting UPF1, a key factor in a variety of RNA decay pathways, and activating the PTEN/PI3K pathway in cancer cells, thereby promoting NSCLC migration and invasion [29]. CAF-derived exosomes also exhibited miR-20a upregulation and promoted NSCLC cell proliferation and chemoresistance via PTEN downregulation following activation of the PI3K/AKT pathway [30]. Cancer cell-derived TGF-β1 activates miR-21 expression in LNFs and induces differentiation to CAFs, which promote the proliferation of cancer cells through the secretion of calumenin [31]. CAF-specific miR-196a promotes NSCLC progression via CCL2 secretion by directly targeting ANXA1 (the gene for annexin-A1) which has anti-inflammatory properties [32]. LncRNAs also participate in activation of LNFs to CAFs, whereas activated CAFs can change gene expression and secretion characteristics of NSCLC cells through lncRNAs [33]. Liu et al. screened fibroblast-specific lncRNAs using RNA-seq data and identified LINC01614 promoting the secretion of IL-6 from cancer cells that upregulates LINC01614 in CAFs, constituting a feedforward loop between CAFs and NSCLC cells [34].
Together, the dysregulation of exosomal miRNA and lncRNAs is involved in the dynamic crosstalk between CAFs and NSCLC cells.
References
- Denys, H.; Derycke, L.; Hendrix, A.; Westbroek, W.; Gheldof, A.; Narine, K.; Pauwels, P.; Gespach, C.; Bracke, M.; De Wever, O. Differential impact of TGF-beta and EGF on fibroblast differentiation and invasion reciprocally promotes colon cancer cell invasion. Cancer Lett. 2008, 266, 263–274.
- Shintani, Y.; Fujiwara, A.; Kimura, T.; Kawamura, T.; Funaki, S.; Minami, M.; Okumura, M. IL-6 Secreted from Cancer-Associated Fibroblasts Mediates Chemoresistance in NSCLC by Increasing Epithelial-Mesenchymal Transition Signaling. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 1482–1492.
- Hasegawa, T.; Yashiro, M.; Nishii, T.; Matsuoka, J.; Fuyuhiro, Y.; Morisaki, T.; Fukuoka, T.; Shimizu, K.; Shimizu, T.; Miwa, A.; et al. Cancer-associated fibroblasts might sustain the stemness of scirrhous gastric cancer cells via transforming growth factor-beta signaling. Int. J. Cancer 2014, 134, 1785–1795.
- Erez, N.; Truitt, M.; Olson, P.; Arron, S.T.; Hanahan, D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell 2010, 17, 135–147.
- Li, J.; Guan, J.; Long, X.; Wang, Y.; Xiang, X. mir-1-mediated paracrine effect of cancer-associated fibroblasts on lung cancer cell proliferation and chemoresistance. Oncol. Rep. 2016, 35, 3523–3531.
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct. Target. Ther. 2021, 6, 218.
- Shintani, Y.; Abulaiti, A.; Kimura, T.; Funaki, S.; Nakagiri, T.; Inoue, M.; Sawabata, N.; Minami, M.; Morii, E.; Okumura, M. Pulmonary fibroblasts induce epithelial mesenchymal transition and some characteristics of stem cells in non-small cell lung cancer. Ann. Thorac. Surg. 2013, 96, 425–433.
- Abulaiti, A.; Shintani, Y.; Funaki, S.; Nakagiri, T.; Inoue, M.; Sawabata, N.; Minami, M.; Okumura, M. Interaction between non-small-cell lung cancer cells and fibroblasts via enhancement of TGF-beta signaling by IL-6. Lung Cancer 2013, 82, 204–213.
- Erez, N.; Glanz, S.; Raz, Y.; Avivi, C.; Barshack, I. Cancer associated fibroblasts express pro-inflammatory factors in human breast and ovarian tumors. Biochem. Biophys. Res. Commun. 2013, 437, 397–402.
- Wang, L.; Cao, L.; Wang, H.; Liu, B.; Zhang, Q.; Meng, Z.; Wu, X.; Zhou, Q.; Xu, K. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget 2017, 8, 76116–76128.
- Iwai, M.; Tulafu, M.; Togo, S.; Kawaji, H.; Kadoya, K.; Namba, Y.; Jin, J.; Watanabe, J.; Okabe, T.; Hidayat, M.; et al. Cancer-associated fibroblast migration in non-small cell lung cancers is modulated by increased integrin alpha11 expression. Mol. Oncol. 2021, 15, 1507–1527.
- Shintani, Y.; Okimura, A.; Sato, K.; Nakagiri, T.; Kadota, Y.; Inoue, M.; Sawabata, N.; Minami, M.; Ikeda, N.; Kawahara, K.; et al. Epithelial to mesenchymal transition is a determinant of sensitivity to chemoradiotherapy in non-small cell lung cancer. Ann. Thorac. Surg. 2011, 92, 1794–1804; discussion 1804.
- Tao, L.; Huang, G.; Wang, R.; Pan, Y.; He, Z.; Chu, X.; Song, H.; Chen, L. Cancer-associated fibroblasts treated with cisplatin facilitates chemoresistance of lung adenocarcinoma through IL-11/IL-11R/STAT3 signaling pathway. Sci. Rep. 2016, 6, 38408.
- Kanzaki, R.; Naito, H.; Kise, K.; Takara, K.; Eino, D.; Minami, M.; Shintani, Y.; Funaki, S.; Kawamura, T.; Kimura, T.; et al. Gas6 derived from cancer-associated fibroblasts promotes migration of Axl-expressing lung cancer cells during chemotherapy. Sci. Rep. 2017, 7, 10613.
- Antony, J.; Huang, R.Y. AXL-Driven EMT State as a Targetable Conduit in Cancer. Cancer Res. 2017, 77, 3725–3732.
- Wang, L.; Li, X.; Ren, Y.; Geng, H.; Zhang, Q.; Cao, L.; Meng, Z.; Wu, X.; Xu, M.; Xu, K. Cancer-associated fibroblasts contribute to cisplatin resistance by modulating ANXA3 in lung cancer cells. Cancer Sci. 2019, 110, 1609–1620.
- Zhang, Q.; Yang, J.; Bai, J.; Ren, J. Reverse of non-small cell lung cancer drug resistance induced by cancer-associated fibroblasts via a paracrine pathway. Cancer Sci. 2018, 109, 944–955.
- De, P.; Aske, J.; Sulaiman, R.; Dey, N. Bete Noire of Chemotherapy and Targeted Therapy: CAF-Mediated Resistance. Cancers 2022, 14, 1519.
- Ferrara, M.G.; Di Noia, V.; D’Argento, E.; Vita, E.; Damiano, P.; Cannella, A.; Ribelli, M.; Pilotto, S.; Milella, M.; Tortora, G.; et al. Oncogene-Addicted Non-Small-Cell Lung Cancer: Treatment Opportunities and Future Perspectives. Cancers 2020, 12, 1196.
- Pellinen, T.; Paavolainen, L.; Martin-Bernabe, A.; Papatella Araujo, R.; Strell, C.; Mezheyeuski, A.; Backman, M.; La Fleur, L.; Bruck, O.; Sjolund, J.; et al. Fibroblast subsets in non-small cell lung cancer: Associations with survival, mutations, and immune features. J. Natl. Cancer Inst. 2022, djac178.
- Choe, C.; Shin, Y.S.; Kim, C.; Choi, S.J.; Lee, J.; Kim, S.Y.; Cho, Y.B.; Kim, J. Crosstalk with cancer-associated fibroblasts induces resistance of non-small cell lung cancer cells to epidermal growth factor receptor tyrosine kinase inhibition. Onco Targets Ther. 2015, 8, 3665–3678.
- Yi, Y.; Zeng, S.; Wang, Z.; Wu, M.; Ma, Y.; Ye, X.; Zhang, B.; Liu, H. Cancer-associated fibroblasts promote epithelial-mesenchymal transition and EGFR-TKI resistance of non-small cell lung cancers via HGF/IGF-1/ANXA2 signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 793–803.
- Yoshida, T.; Ishii, G.; Goto, K.; Neri, S.; Hashimoto, H.; Yoh, K.; Niho, S.; Umemura, S.; Matsumoto, S.; Ohmatsu, H.; et al. Podoplanin-positive cancer-associated fibroblasts in the tumor microenvironment induce primary resistance to EGFR-TKIs in lung adenocarcinoma with EGFR mutation. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 642–651.
- Shien, K.; Papadimitrakopoulou, V.A.; Ruder, D.; Behrens, C.; Shen, L.; Kalhor, N.; Song, J.; Lee, J.J.; Wang, J.; Tang, X.; et al. JAK1/STAT3 Activation through a Proinflammatory Cytokine Pathway Leads to Resistance to Molecularly Targeted Therapy in Non-Small Cell Lung Cancer. Mol. Cancer Ther. 2017, 16, 2234–2245.
- Wang, Q.; Yang, S.; Wang, K.; Sun, S.Y. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. J. Hematol. Oncol. 2019, 12, 63.
- Apicella, M.; Giannoni, E.; Fiore, S.; Ferrari, K.J.; Fernandez-Perez, D.; Isella, C.; Granchi, C.; Minutolo, F.; Sottile, A.; Comoglio, P.M.; et al. Increased Lactate Secretion by Cancer Cells Sustains Non-cell-autonomous Adaptive Resistance to MET and EGFR Targeted Therapies. Cell Metab. 2018, 28, 848–865.e846.
- Shen, H.; Yu, X.; Yang, F.; Zhang, Z.; Shen, J.; Sun, J.; Choksi, S.; Jitkaew, S.; Shu, Y. Reprogramming of Normal Fibroblasts into Cancer-Associated Fibroblasts by miRNAs-Mediated CCL2/VEGFA Signaling. PLoS Genet. 2016, 12, e1006244.
- Shen, Z.Z.; Qin, X.; Yan, M.; Li, R.R.; Chen, G.; Zhang, J.J.; Chen, W.T. Cancer-associated fibroblasts promote cancer cell growth through a miR-7-RASSF2-PAR-4 axis in the tumor microenvironment. Oncotarget 2017, 8, 1290–1303.
- Yang, F.; Yan, Y.; Yang, Y.; Hong, X.; Wang, M.; Yang, Z.; Liu, B.; Ye, L. MiR-210 in exosomes derived from CAFs promotes non-small cell lung cancer migration and invasion through PTEN/PI3K/AKT pathway. Cell. Signal. 2020, 73, 109675.
- Shi, L.; Zhu, W.; Huang, Y.; Zhuo, L.; Wang, S.; Chen, S.; Zhang, B.; Ke, B. Cancer-associated fibroblast-derived exosomal microRNA-20a suppresses the PTEN/PI3K-AKT pathway to promote the progression and chemoresistance of non-small cell lung cancer. Clin. Transl. Med. 2022, 12, e989.
- Kunita, A.; Morita, S.; Irisa, T.U.; Goto, A.; Niki, T.; Takai, D.; Nakajima, J.; Fukayama, M. MicroRNA-21 in cancer-associated fibroblasts supports lung adenocarcinoma progression. Sci. Rep. 2018, 8, 8838.
- Lee, S.; Hong, J.H.; Kim, J.S.; Yoon, J.S.; Chun, S.H.; Hong, S.A.; Kim, E.J.; Kang, K.; Lee Kang, J.; Ko, Y.H.; et al. Cancer-associated fibroblasts activated by miR-196a promote the migration and invasion of lung cancer cells. Cancer Lett. 2021, 508, 92–103.
- Ti, W.; Wang, J.; Cheng, Y. The Interaction Between Long Non-Coding RNAs and Cancer-Associated Fibroblasts in Lung Cancer. Front. Cell Dev. Biol. 2021, 9, 714125.
- Liu, T.; Han, C.; Fang, P.; Ma, Z.; Wang, X.; Chen, H.; Wang, S.; Meng, F.; Wang, C.; Zhang, E.; et al. Cancer-associated fibroblast-specific lncRNA LINC01614 enhances glutamine uptake in lung adenocarcinoma. J. Hematol. Oncol. 2022, 15, 141.
More
