Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Conner Chen and Version 2 by Conner Chen.
Iodinated X-ray contrast media (ICM) as emerging micropollutants have attracted considerable attention due to their high detected concentration in water systems. It results in environmental issues partly due to the formation of toxic by-products during the disinfection process in water treatment.
- iodinated X-ray contrast media
- depollution
- byproducts
1. Introduction
The continuous increase in the human population has created a comparable growth in the demand for water resources. In such an urgent situation, protecting our aquatic environment to achieve a comprehensive sustainable development strategy is one of the most essential environmental topics of the past decades. It has been shown that abounding compounds can enter the environment, such as pharmaceuticals [1][2][3], disinfectants, and other contaminants [4][5][6]. One of the most important factors contributing to this phenomenon is hospitals’ effluents as a source of contaminants discharge, such as specific iodinated X-ray contrast media (ICM) [7][8][9], antitumor agents [10][11] as well as antibiotics [12][13].
Since the discovery of X-ray radiation by Wilhelm C. Rogentgen in 1895, the diagnostic imaging procedure has become a routine part of medicine due to the possible external visualization of the internal anatomic structures of a patient without surgery [14]. Suspensions of barium sulfate have been applied for radiographic imaging since 1910. However, the marked radiodensity and poor intrinsic affinity for the gastrointestinal mucosa of this agent led to inaccurate diagnoses. In such a background, water-soluble iodinated X-ray contrast media (ICM) are the most extensively used pharmaceuticals for intravascular administration [15].
ICM are derivatives of 2,4,6-triiodobenzoic acid with polar carboxyl and hydroxyl moieties in their chains. Some of them are ionic, i.e. have one or several free carboxyl groups, and others are amide derivatives. They have all three iodine atoms in their chemical structure, which are responsible for the absorption of X-rays.
As ICM have been widely used for more than 40 years, their production is well-controlled. However, their synthesis is still the subject of high investigations to make it greener [16] and more efficient [17][18]. The recovery of the iodine atoms in industrial wastewater is also well-studied as it can reduce the cost of the overall production process [19][20].
2. ICM in the Environment
2.1. Effluents Polluted by ICM
From the internet site of the French National College of Medical Pharmacology (https://pharmacomedicale.org, accessed on 28 December 2022), 1.5 million contrast media injections were notified in France in 2018 and the most used were iodinated contrast media. Moreover, in the nineties, the annual consumption of ICM worldwide is estimated to have reached a value of 3.5 × 106 kg, since they are administered intravascularly in very high doses (up to 200 g ICM, corresponding to approximately 100 g iodine per application) [21]. In addition, ICM has an average half-life in the human body of around 2 h, and 75% of the administered dose is excreted via urine or feces into the hospital wastewater within 4h and nearly 100% within 24 h [22]. They were identified as a major source of increased absorbable organic halogen (AOX) concentrations in hospital sewage water [23]. In a large part of the region, hospital wastewater is not treated separately and is finally discharged into the local sewage systems together with domestic wastewater. Even if wastewater treatment plants (WWTP) can eliminate most pharmaceutical chemicals during the treatment process, various treatment systems are required for different contaminants in order to achieve efficient removal. Pollutants with polar characters can easily bypass the water purification process and so reach surface waters with a potential impact on the ecosystem and human health [24]. The n-octanol/water partition coefficient of ICM (Table 1) characterizes them as very hydrophilic.Table 1. Chemical structure and physicochemical properties of ICM.
| Diatrizoic Acid | Iohexol | Iopamidol | Iopromide | Ioxithalamic Acid | Iomeprol | |
|---|---|---|---|---|---|---|
| Structure | 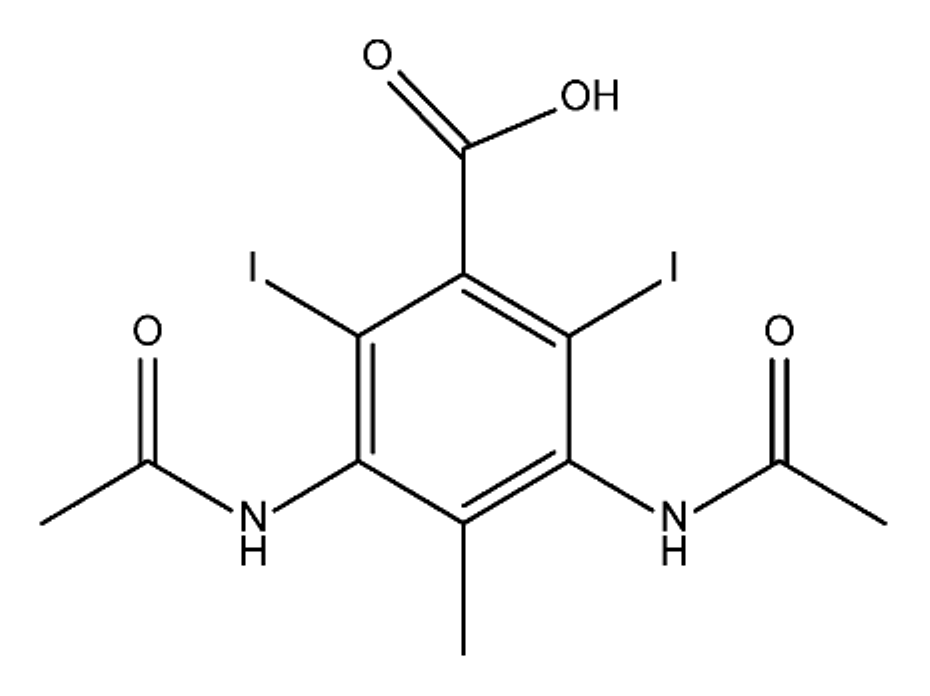 |
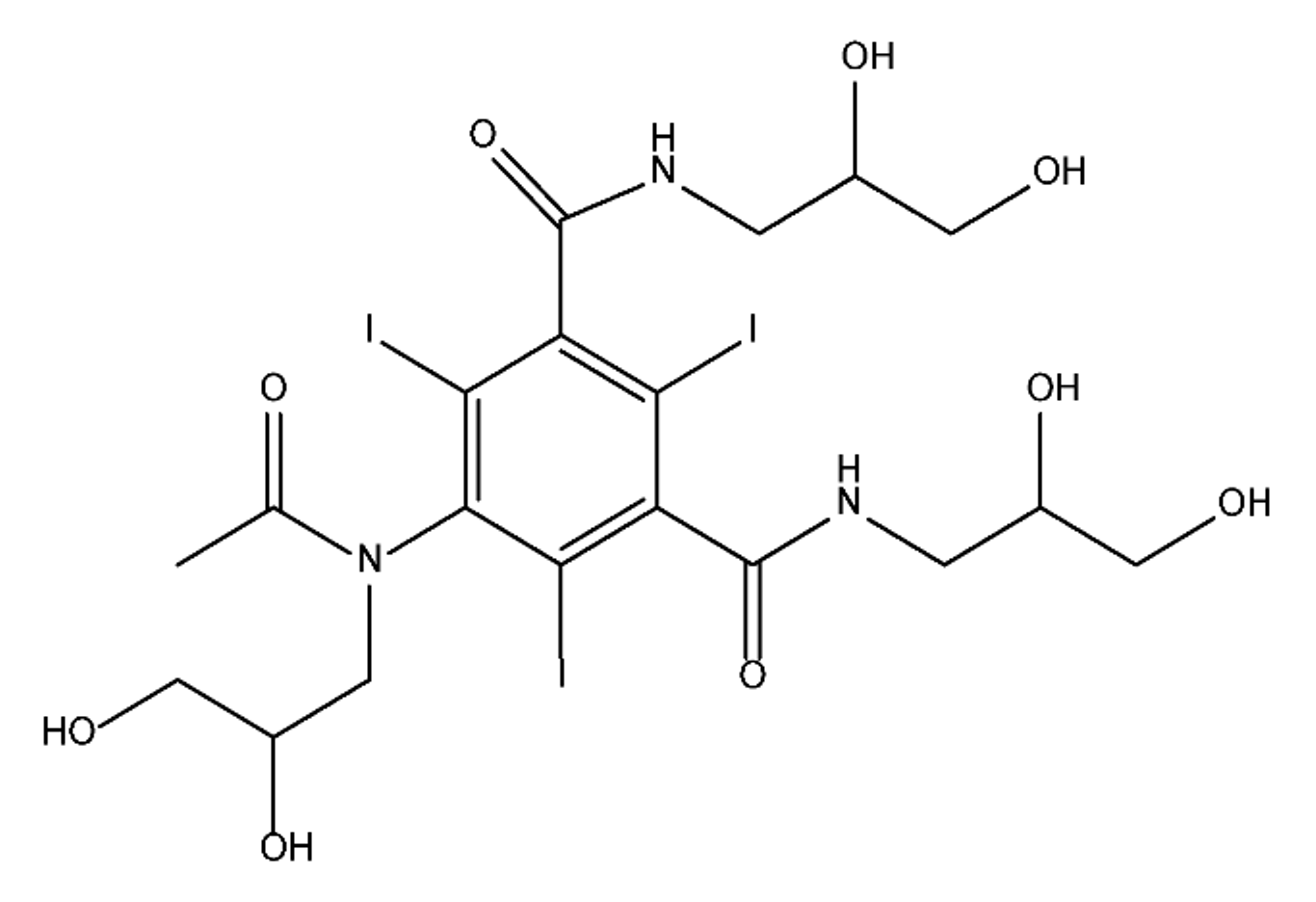 |
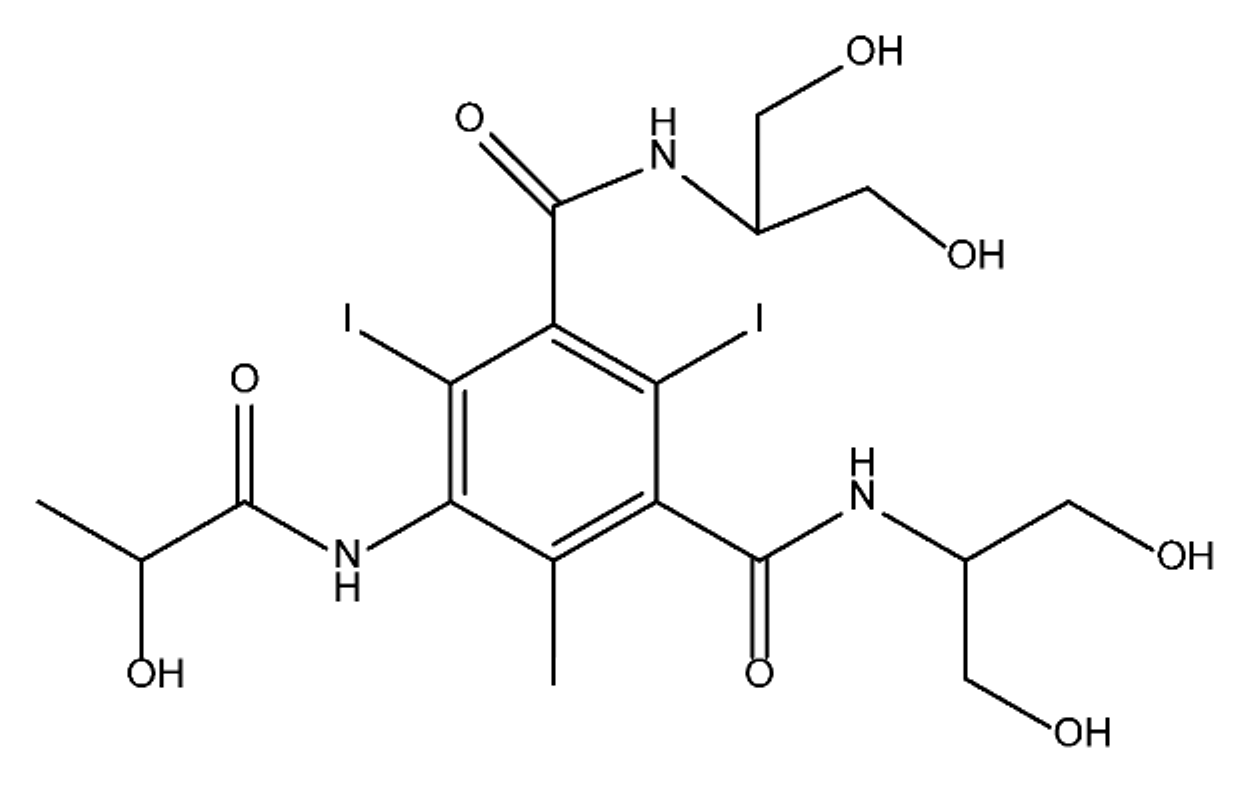 |
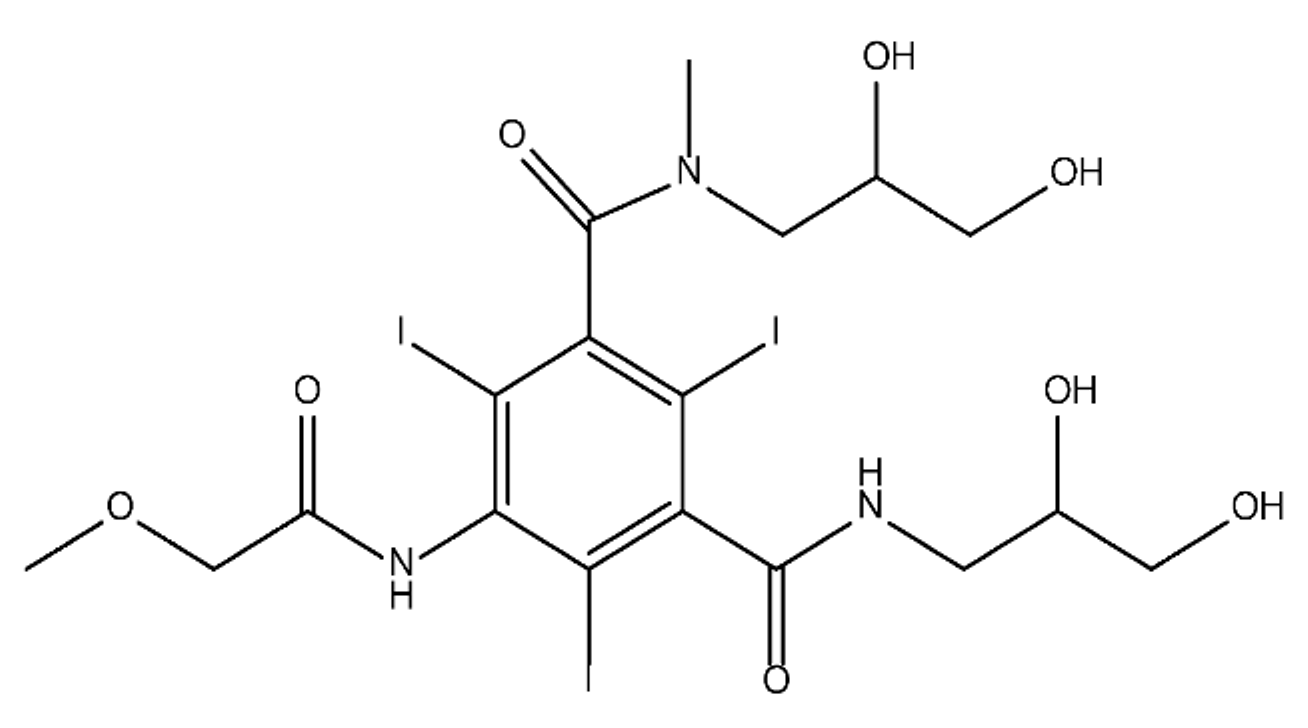 |
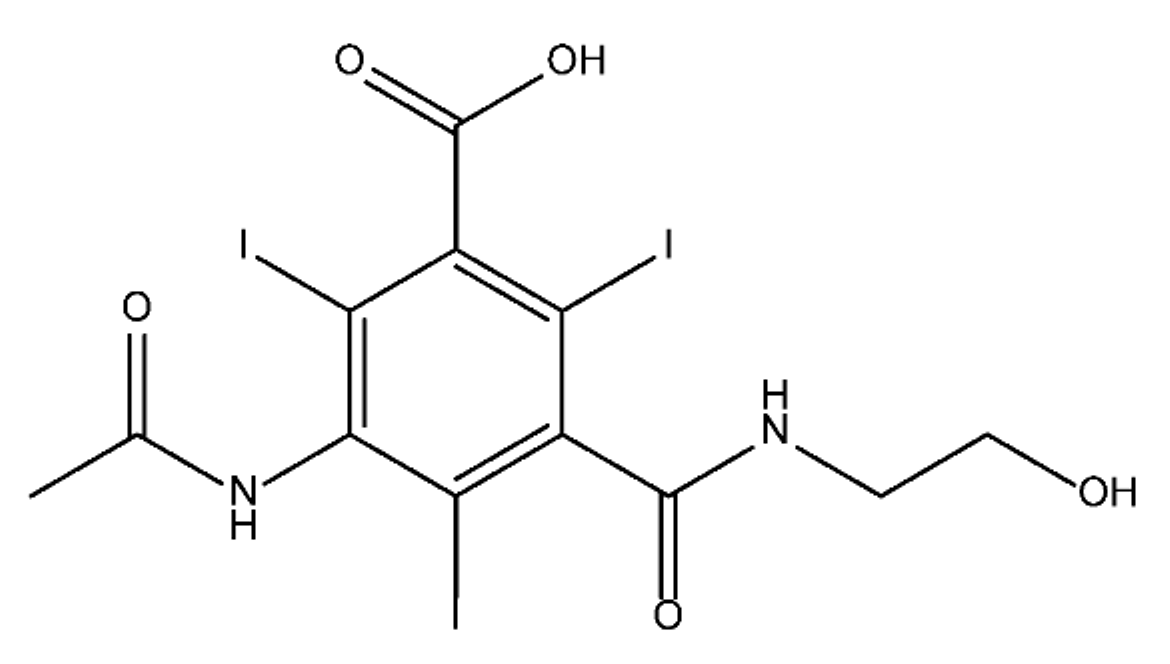 |
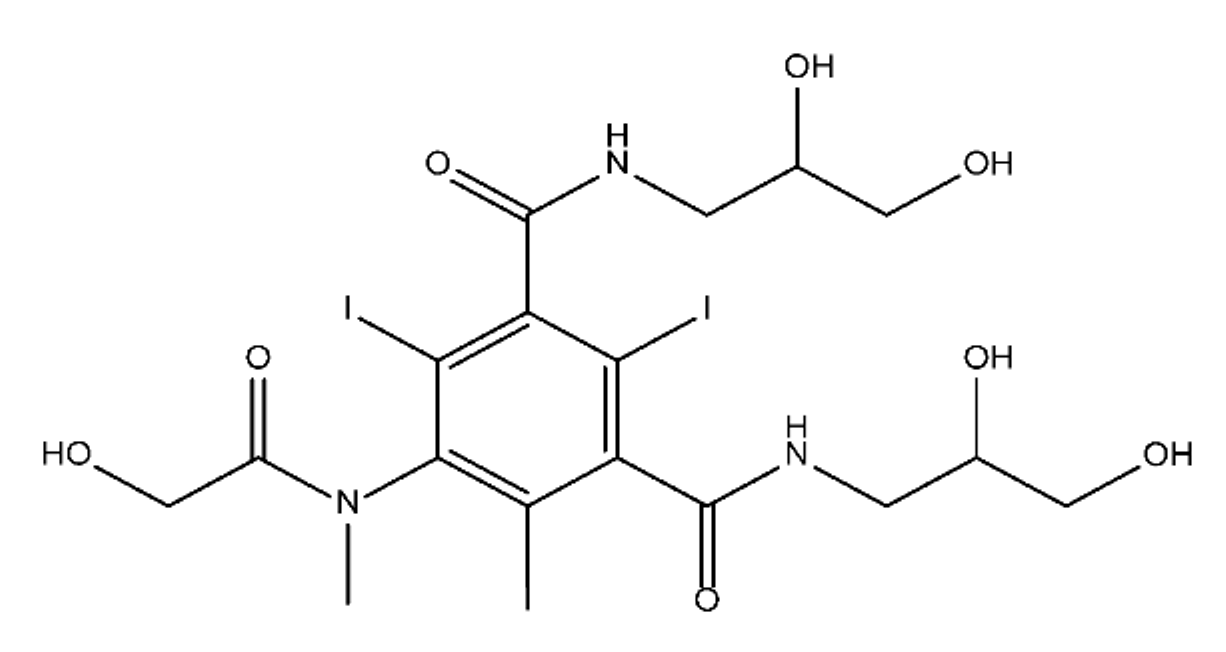 |
| Log Kow a | 1.8 | −3 | −2.4 | −2.1 | 1.2 | −2.3 |
| Ionicity | Ionic | Nonionic | Nonionic | Nonionic | Ionic | Nonionic |
| pKa | 2.17 | 11.73 | 11.00 | 11.09 | 2.13 | 11.73 |
| Concentrations b (μg L−1) | ||||||
| Hospital wastewater | 17.1–61 | 0.07–3810 | 0.03–2599 | 0.008–3.2 | 15–550 | 0.05–2400 |
| Surface water | 0.032–4.55 | 0.01–1.326 | 0.008–3.2 | 0.01–13 | 0.01–0.438 | 0.023–6.1 |
| Ground water | 0.02–9.6 | 0.003–0.187 | 0.006–0.47 | 0.003–0.687 | 0.204 | 0.003–1.655 |
| Drinking water | 0.0009–1.2 | 0.001–0.034 | 0.02–0.27 | 0.0005–0.084 | - | 0.0013–0.034 |
a [8] b Results in Europe from 2001 [25].
As a consequence, a large amount of literature has revealed that ICM have been detected in our living waters. For example, Seitz et al. [26] determined ICM concentrations in the Danube river through a monitoring programmer. Their study revealed that the maximum concentrations of ICM (over 500 ng L−1 for diatrizoic acid and iopamidol) were found in samples taken from downstream of the Neu-Ulm metropolitan area of Germany. A similar situation was found in Galicia, Spain [27] and in the southwestern United States [28]. In the same way, in China, total ICM concentrations around 160 and 97.4 ng L−1 were found in the Huangpu River and the Tahu Lake, respectively; higher concentrations (about 430 ng L−1) of iodinated tri-halomethane, an ICM oxidation product, were determined in local wastewater plant from these places [29].
In a recent review, Sengar and Vijayanandan summarize data from 51 articles from 2001 on the occurrence of ICM in hospital wastewater, surface water, groundwater, and drinking water in North America, Asia, and Europe [8]. Table 1 summarizes the concentration range found in Europe for diatrizoate, iohexol, iopamidol, iopromide, ioxithalamic acid, and iomeprol. Concentrations from 0.01 to 3800 μg L−1 were found in hospital wastewater, with the highest reached concentrations for iohexol, iopamidol, and iomeprol. In surface and groundwater, the concentrations were lower ranging from 10−3 to 10 μg L−1 and <0.02 μg L−1 for drinking water.
Considering the high concentrations in hospital wastewater, the MERK’MAL project suggested urine collection in order to reduce the ICM environmental impact on the Ruhr River [30]. The collection was conducted on four medical facilities using daily ICM via urine bags. This concept allowed an ICM reduction in the range of 20–30% in the tested wastewater treatment plants.
2.2. Ecotoxicology of ICM
Pharmaceuticals are designed to target specific metabolic and molecular pathways in humans and animals, but they often have important side effects too. When introduced into the environment, they may affect the same pathways in animals having identical or similar target organs, tissues, cells, or biomolecules [31]. For ICM acute ecotoxicology, even if it is usually reversible, ICM can lead to contrast-induced nephropathy (CIN), which is associated with longer hospitalization time, the need for dialysis, higher incidence of later cardiovascular events, and higher mortality [32]. However, in short-term toxicity tests with bacteria (Vibrio fisheri, Pseudomonas putida), algae (Scenedesmus subspicatus), crustaceans (Daphnia magna), and fish (Danio rerio, Leuciscus idus), no toxic effects were detected at the highest tested concentration of 10 g L−1 according to the study of Steger-Hartmann [33]. Ecotoxicity investigation gave indications of acute effects in vivo in organisms after short-term exposure, but only rarely after chronic exposures. Oleksy-Frenzel et al. [34] have shown that ICM may significantly contribute to the burden of absorbable organic halogens (AOX), which can cause a severe underestimation of pollution in municipal wastewater. Considering disinfection processes in water treatment, as most ICM have an amino group in their molecular structure, the production of some nitrogenous byproducts such as haloacetonitrile; haloacetamides occurs and is problematic due to their toxicity [35]. Moreover, iodinated trihalomethane, another toxic ICM byproduct can be produced from the ICM reaction with disinfecting agent [36]. In the case of Iopamidol, the formation of toxic iodinated trihalomethane can be reduced during the chlorination step after a pre-oxidation with Fe(VI) at pH 9. In conclusion, the occurrence of ICM in the environment has been largely observed over the world. Although their toxicity is low, their presence during wastewater treatment and disinfection process can lead to the formation of toxic by-products. It is therefore a major concern that has to be solved. Their total mineralization by concentrated wastewater treatment can be a solution.References
- Nieto-Juárez, J.I.; Torres-Palma, R.A.; Botero-Coy, A.; Hernández, F. Pharmaceuticals and environmental risk assessment in municipal wastewater treatment plants and rivers from Peru. Environ. Int. 2021, 155, 106674.
- Anh, H.Q.; Le, T.P.Q.; Da Le, N.; Lu, X.X.; Duong, T.T.; Garnier, J.; Rochelle-Newall, E.; Zhang, S.; Oh, N.-H.; Oeurng, C.; et al. Antibiotics in surface water of East and Southeast Asian countries: A focused review on contamination status, pollution sources, potential risks, and future perspectives. Sci. Total. Environ. 2020, 764, 142865.
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155.
- Arman, N.Z.; Salmiati, S.; Aris, A.; Salim, M.R.; Nazifa, T.H.; Muhamad, M.S.; Marpongahtun, M. A Review on Emerging Pollutants in the Water Environment: Existences, Health Effects and Treatment Processes. Water 2021, 13, 3258.
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging contaminants of high concern for the environment: Current trends and future research. Environ. Res. 2022, 207, 112609.
- Kümmerer, K. Drugs in the environment: Emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources–a review. Chemosphere 2001, 45, 957–969.
- Dekker, H.M.; Stroomberg, G.J.; Prokop, M. Tackling the increasing contamination of the water supply by iodinated contrast media. Insights into Imaging 2022, 13, 30.
- Sengar, A.; Vijayanandan, A. Comprehensive review on iodinated X-ray contrast media: Complete fate, occurrence, and formation of disinfection byproducts. Sci. Total. Environ. 2021, 769, 144846.
- Pauwels, B.; Verstraete, W. The treatment of hospital wastewater: An appraisal. J. Water Health 2006, 4, 405–416.
- Khan, M.T.; Shah b, I.A.; Ihsanullah, I.; Naushad, M.; Ali, S.; Shah, S.H.A.; Mohammad, A.W. Hospital wastewater as a source of environmental contamination: An overview of management practices, environmental risks, and treatment processes. J. Water Process Eng. 2021, 41, 101990.
- Ternes, T.A.; Bonerz, M.; Herrmann, N.; Loffler, D.; Keller, E.; Lacida, B.B.; Alder, A.C. Determination of pharmaceuticals, iodinated contrast media and musk fragrances in sludge by LC/tandem MS and GC/MS. J. Chromatogr. A 2005, 1067, 213–223.
- Harrower, J.; McNaughtan, M.; Hunter, C.; Hough, R.; Zhang, Z.; Helwig, K. Chemical Fate and Partitioning Behavior of Antibiotics in the Aquatic Environment—A Review. Environ. Toxicol. Chem. 2021, 40, 3275–3298.
- Giger, W.; Alder, A.C.; Golet, E.M.; Kohler, H.-P.E.; McArdell, C.S.; Molnar, E.; Siegrist, H.; Suter, M.J.-F. Occurrence and Fate of Antibiotics as Trace Contaminants in Wastewaters, Sewage Sludges, and Surface Waters. CHIMIA 2003, 57, 485.
- Yu, S.-B.; Watson, A.D. Metal-based X-ray contrast media. Chem. Rev. 1999, 99, 2353–2378.
- Estep, K.G.; Josef, K.A.; Bacon, E.R.; Illig, C.R.; Toner, J.L.; Mishra, D.; Blazak, W.F.; Miller, D.M.; Johnson, D.K.; Allen, J.M.; et al. 1,3,5-Trialkyl-2,4,6-triiodobenzenes: Novel X-ray Contrast Agents for Gastrointestinal Imaging. J. Med. Chem. 2000, 43, 1940–1948.
- Barge, A.; Baricco, F.; Cravotto, G.; Fretta, R.; Lattuada, L. Mechanochemistry Applied to the Synthesis of X-ray Contrast Agent. ACS Sustain. Chem. Eng. 2020, 8, 12825–12830.
- Haaland, T.; Sydnes, L.K. Formation of N,O-Acetals in the Production of X-ray Contrast Agents. Org. Process Res. Dev. 2014, 18, 1181–1190.
- Haarr, M.B.; Lindbäck, E.; Håland, T.; Sydnes, M.O. Synthesis of an Alleged Byproduct Precursor in Iodixanol Preparation. ACS Omega 2018, 3, 7344–7349.
- Radjenovic, J.; Flexer, V.; Donose, B.C.; Sedlak, D.L.; Keller, J. Removal of the X-ray Contrast Media Diatrizoate by Electrochemical Reduction and Oxidation. Environ. Sci. Technol. 2013, 47, 13686–13694.
- Zhang, W.; Soutrel, I.; Amrane, A.; Fourcade, F.; Geneste, F. Electrochemical Processes Coupled to a Biological Treatment for the Removal of Iodinated X-ray Contrast Media Compounds. Front. Chem. 2020, 8, 646.
- Mendoza, A.; Aceña, J.; Pérez, S.; de Alda, M.L.; Barceló, D.; Gil, A.; Valcárcel, Y. Pharmaceuticals and iodinated contrast media in a hospital wastewater: A case study to analyse their presence and characterise their environmental risk and hazard. Environ. Res. 2015, 140, 225–241.
- Weissbrodt, D.G.; Kovalova, L.; Ort, C.; Pazhepurackel, V.; Moser, R.; Hollender, J.; Siegrist, H.; McArdell, C.S. Mass Flows of X-ray Contrast Media and Cytostatics in Hospital Wastewater. Environ. Sci. Technol. 2009, 43, 4810–4817.
- Kümmerer, K.; Erbe, T.; Gartiser, S.; Brinker, L. AOX—Emiissions from hospitals into municipal waste water. Chemosphere 1998, 36, 2437–2445.
- Pérez, S.; Barceló, D. Fate and occurrence of X-ray contrast media in the environment. Anal. Bioanal. Chem. 2006, 387, 1235–1246.
- Seitz, W.; Weber, W.H.; Jiang, J.-Q.; Lloyd, B.J.; Maier, M.; Maier, D.; Schulz, W. Monitoring of iodinated X-ray contrast media in surface water. Chemosphere 2006, 64, 1318–1324.
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; García-Jares, C.; Rodríguez, I.; Gómez, M.; Ternes, T. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 2004, 38, 2918–2926.
- Drewes, J.E.; Fox, P.; Jekel, M. Occurrence of iodinated X-ray contrast media in domestic effluents and their fate during indirect potable reuse. J. Environ. Sci. Health Part A 2001, 36, 1633–1645.
- Xu, Z.; Li, X.; Hu, X.; Yin, D. Distribution and relevance of iodinated X-ray contrast media and iodinated trihalomethanes in an aquatic environment. Chemosphere 2017, 184, 253–260.
- Strehl, C.; Thoene, V.; Heymann, L.; Schwesig, D.; Boergers, A.; Bloser, M.; Fligge, F.; Merkel, W.; Tuerk, J. Cost-effective reduction of micro pollutants in the water cycle—Case study on iodinated contrast media. Sci. Total Environ. 2019, 688, 10–17.
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159.
- Quiros, Y.; Sánchez-González, P.D.; López-Hernández, F.J.; Morales, A.I.; López-Novoa, J.M. Cardiotrophin-1 Administration Prevents the Renal Toxicity of Iodinated Contrast Media in Rats. Toxicol. Sci. 2013, 132, 493–501.
- Steger-Hartmann, T.; Länge, R.; Schweinfurth, H. Environmental Risk Assessment for the Widely Used Iodinated X-Ray Contrast Agent Iopromide (Ultravist). Ecotoxicol. Environ. Saf. 1999, 42, 274–281.
- Oleksy-Frenzel, J.; Wischnack, S.; Jekel, M. Application of ion-chromatography for the determination of the organic-group parameters AOCl, AOBr and AOI in water. Anal. Bioanal. Chem. 2000, 366, 89–94.
- Lopez-Prieto, I.J.; Park, M.; AzadiAghdam, M.; Pan, H.; Jones, S.L.; Snyder, S.A. Formation and control of disinfection by-products from iodinated contrast media attenuation through sequential treatment processes of ozone-low pressure ultraviolet light followed by chlorination. Chemosphere 2021, 278, 130394.
- Li, M.; Zhang, T.-Y.; Xu, B.; Hu, C.-Y.; Dong, Z.-Y.; Wang, Z.; Tang, Y.-L.; Yu, S.-L.; Pan, Y.; Xian, Q. Iodinated trihalomethanes formation in iopamidol-contained water during ferrate/chlor(am)ination treatment. Chemosphere 2021, 272, 129568.
- Ternes, T.A.; Hirsch, R. Occurrence and Behavior of X-ray Contrast Media in Sewage Facilities and the Aquatic Environment. Environ. Sci. Technol. 2000, 34, 2741–2748.
More
