The ability to precisely monitor the intracellular temperature directly contributes to the essential understanding of biological metabolism, intracellular signaling, thermogenesis, and respiration. The intracellular heat generation and its measurement can also assist in the prediction of the pathogenesis of chronic diseases. Intracellular thermometry without altering the biochemical reactions and cellular membrane damage is challenging, requiring appropriately biocompatible, nontoxic, and efficient biosensors. Bright, photostable, and functionalized fluorescent nanodiamonds (FNDs) have emerged as excellent probes for intracellular thermometry and magnetometry with the spatial resolution on a nanometer scale. The temperature and magnetic field-dependent luminescence of naturally occurring defects in diamonds are key to high-sensitivity biosensing applications. Alterations in the surface chemistry of FNDs and conjugation with polymer, metallic, and magnetic nanoparticles have opened vast possibilities for drug delivery, diagnosis, nanomedicine, and magnetic hyperthermia. The possibilities and outcomes of using AI strategies recommended for early stage disease diagnosis and imaging are discuessed.
- fluorescent nanodiamonds
- AI
- biosensing
- bioimaging
1. Introduction
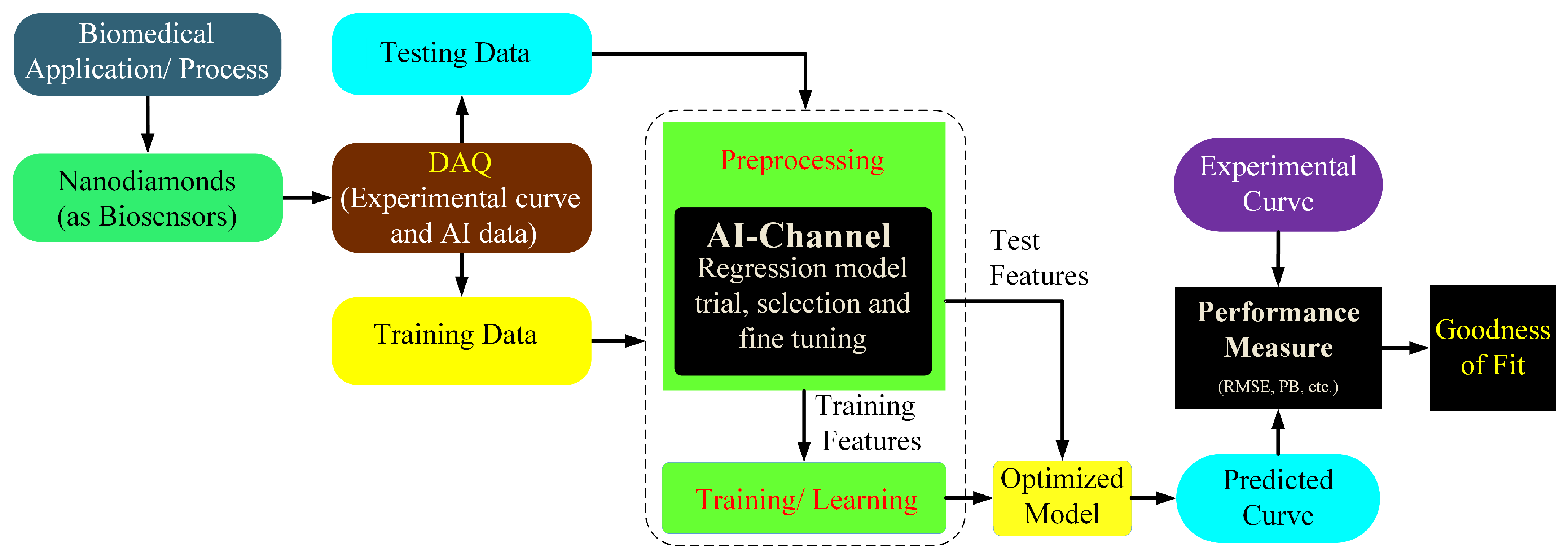
2. Application of Functionalized Fluorescent NDanodiamonds Bioimaging with Artificial Intelligence
References
- Ozawa, H.; Hatano, Y.; Iwasaki, T.; Harada, Y.; Hatano, M. Formation of perfectly aligned high-density nv centers in (111) cvd-grown diamonds for magnetic field imaging of magnetic particles. Jpn. J. Appl. Phys. 2019, 58, SIIB26.
- Balasubramanian, G.; Neumann, P.; Twitchen, D.; Markham, M.; Kolesov, R.; Mizuochi, N.; Isoya, J.; Achard, J.; Beck, J.; Tissler, J. Ultralong spin coherence time in isotopically engineered diamond. Nat. Mater. 2009, 8, 383–387.
- Stehlik, S.; Varga, M.; Ledinsky, M.; Jirasek, V.; Artemenko, A.; Kozak, H.; Ondic, L.; Skakalova, V.; Argentero, G.; Pennycook, T. Size and purity control of hpht nanodiamonds down to 1 nm. J. Phys. Chem. C 2015, 119, 27708–27720.
- Chen, L.; Miao, X.; Ma, H.; Guo, L.; Wang, Z.; Yang, Z.; Fang, C.; Jia, X. Synthesis and characterization of diamonds with different nitrogen concentrations under high pressure and high temperature conditions. CrystEngComm 2018, 20, 7164–7169.
- Smeltzer, B.; Childress, L.; Gali, A. 13c hyperfine interactions in the nitrogen-vacancy centre in diamond. New J. Phys. 2011, 13, 025021.
- Felton, S.; Edmonds, A.; Newton, M.; Martineau, P.; Fisher, D.; Twitchen, D.; Baker, J. Hyperfine interaction in the ground state of the negatively charged nitrogen vacancy center in diamond. Phys. Rev. B 2009, 79, 075203.
- Antonov, D.; Häußermann, T.; Aird, A.; Roth, J.; Trebin, H.-R.; Müller, C.; McGuinness, L.; Jelezko, F.; Yamamoto, T.; Isoya, J. Statistical investigations on nitrogen-vacancy center creation. Appl. Phys. Lett. 2014, 104, 012105.
- Orwa, J.; Santori, C.; Fu, K.; Gibson, B.; Simpson, D.; Aharonovich, I.; Stacey, A.; Cimmino, A.; Balog, P.; Markham, M. Engineering of nitrogen-vacancy color centers in high purity diamond by ion implantation and annealing. J. Appl. Phys. 2011, 109, 083530.
- Pezzagna, S.; Naydenov, B.; Jelezko, F.; Wrachtrup, J.; Meijer, J. Creation efficiency of nitrogen-vacancy centres in diamond. New J. Phys. 2010, 12, 065017.
- Chen, S.; Li, W.; Zheng, X.; Yu, P.; Wang, P.; Sun, Z.; Xu, Y.; Jiao, D.; Ye, X.; Cai, M. Immunomagnetic microscopy of tumor tissues using quantum sensors in diamond. Proc. Natl. Acad. Sci. USA 2022, 119, e2118876119.
- Cui, F.; Yue, Y.; Zhang, Y.; Zhang, Z.; Zhou, H.S. Advancing biosensors with machine learning. ACS Sens. 2020, 5, 3346–3364.
- Dolenko, T.A.; Burikov, S.A.; Vervald, A.M.; Vlasov, I.I.; Dolenko, S.A.; Laptinskiy, K.A.; Rosenholm, J.M.; Shenderova, O.A. Optical imaging of fluorescent carbon biomarkers using artificial neural networks. J. Biomed. Opt. 2014, 19, 117007.
- Burikov, S.A.; Vervald, A.M.; Vlasov, I.I.; Dolenko, S.A.; Laptinskiy, K.; Dolenko, T.A. Use of neural network algorithms for elaboration of fluorescent biosensors on the base of nanoparticles. Opt. Mem. Neural Netw. 2013, 22, 156–165.
- Loh, K.P.; Ho, D.; Chiu, G.N.C.; Leong, D.T.; Pastorin, G.; Chow, E.K.H. Clinical applications of carbon nanomaterials in diagnostics and therapy. Adv. Mater. 2018, 30, 1802368.
- Blasiak, A.; Khong, J.; Kee, T. Curate. Ai: Optimizing personalized medicine with artificial intelligence. SLAS Technol. 2020, 25, 95–105.
- Wang, H.; Lee, D.-K.; Chen, K.-Y.; Chen, J.-Y.; Zhang, K.; Silva, A.; Ho, C.-M.; Ho, D. Mechanism-independent optimization of combinatorial nanodiamond and unmodified drug delivery using a phenotypically driven platform technology. ACS Nano 2015, 9, 3332–3344.
- Qureshi, S.A.; Raza, S.E.A.; Hussain, L.; Malibari, A.A.; Nour, M.K.; Rehman, A.u.; Al-Wesabi, F.N.; Hilal, A.M. Intelligent ultra-light deep learning model for multi-class brain tumor detection. Appl. Sci. 2022, 12, 3715.
