Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Da Sun.
Konjac glucomannan (KGM), can not only be applied as a food additive, which greatly improves the taste and flavor of food and extends the shelf life of food but also occupies an important role in T2DM. KGM can extend gastric emptying time, increase satiety, and promote liver glycogen synthesis, and also has the potential to improve intestinal flora and the metabolic system through a variety of molecular pathways in order to positively regulate oxidative stress and immune inflammation, and protect the liver and kidneys.
- konjac glucomannan
- type 2 diabetes mellitus
- FSMP
1. Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic syndrome characterized by decreased insulin sensitivity and insufficient insulin secretion, which is related to the unhealthy metabolism of sugar, fat, amino acids, water, and electrolytes. Genetics [1[1][2],2], insulin abnormalities [3], and metabolic disturbance are key factors contributing to the disease. Nowadays, studies have shown that the gut microbiome may also influence the development of T2DM [4,5][4][5]. More than 422 million people worldwide have been diagnosed with diabetes mellitus, and the disease claims more than 1.6 million lives annually, according to a recent World Health Organization report [6]. T2DM will affect 439 million people worldwide by the year 2030, making up about 90% to 95% of all cases [7,8][7][8]. However, traditional treatment cannot cure it.
T2DM is treated with drugs (such as biguanides, glucosidase inhibitors, and thia-zolidinediones) that manage blood pressure, cholesterol, and blood glucose levels [9]. However, long-term use of pharmaceuticals can lead to adverse effects such as bloating and diarrhea [10], and most individuals with T2DM will eventually require insulin therapy to maintain normal blood sugar levels. In this way, the idea of homology between medicine and food is gradually rising, such as control diets to alleviate the progressive worsening of diabetes [11]. The combination of diet and medicine can control the levels of blood sugar and urine sugar without or with only minimal medicine, especially for patients with mild illness, while those with moderate or severe illnesses even can reduce the amount of medication they require. Among the many therapeutic options studied, there is a growing interest in exploring the therapeutic effects of dietary fiber as a special medical food [12]. Consuming dietary fiber has a multitude of metabolic benefits that are unrelated to changes in weight, including impacts on various metabolic and inflammatory diseases, enhanced insulin sensitivity, and regulation of certain gut hormones. Konjac glucomannan (KGM) is a kind of dietary fiber derived from konjac, that is not easily hydrolyzed by human digestive tract enzymes, and can directly enter the colon and be used by intestinal microorganisms [13]. Long-term consumption can also reduce calories, balance body salt, and improve obesity and diabetes, to improve the sub-health of the human body [14].
Studies have shown that KGM as a sticky dietary fiber can improve subjective satiety and reduce appetite, which is very beneficial for patients with T2DM [15]. Adding KGM as a dietary additive to noodles can not only slow down the aging rate of noodles, but also improve the storage stability of cooked noodles [16]. This diet may have potential long-term health outcomes, such as lower blood lipids, postprandial blood glucose and insulin levels, and the benefits of this blood sugar response are highly dependent on the type of substrate and the dose of KGM [17,18][17][18]. Compared with other dietary fibers (cereal fiber and vegetable fiber), the purified KGM has a single component and is safer for the human body. The amount of dietary fiber per 100 g of other diets (such as soybeans, wheat, and corn) is much lower than that of KGM. In addition, KGM has a wide range of sources (Figure 1), simple extraction process, high efficiency, and considerable price advantages. The most important thing is that in addition to regulating blood sugar, it can also regulate blood lipids, intestinal flora, oxidative stress, immune suppression and so on. However, it should be noted that dietary fiber itself does not have high nutritional value and cannot be used as a long-term staple food. Although KGM cannot be used as a drug in the treatment of disease, it can be used as a special medical food to assist the treatment of type 2 diabetes mellitus. Since increasing evidence linking the consumption of highly processed foods with an increased risk of noncommunicable diseases poses a public health challenge [19,20[19][20][21],21], here wresearchers review the structure and physicochemical properties of naturally extracted KGM, discuss its benefits on various physiological and biochemical indexes of type 2 diabetes mellitus, and explain the effects of related mechanisms and pathways, in order to reveal its great potential in food for a special medical purpose (FSMP).
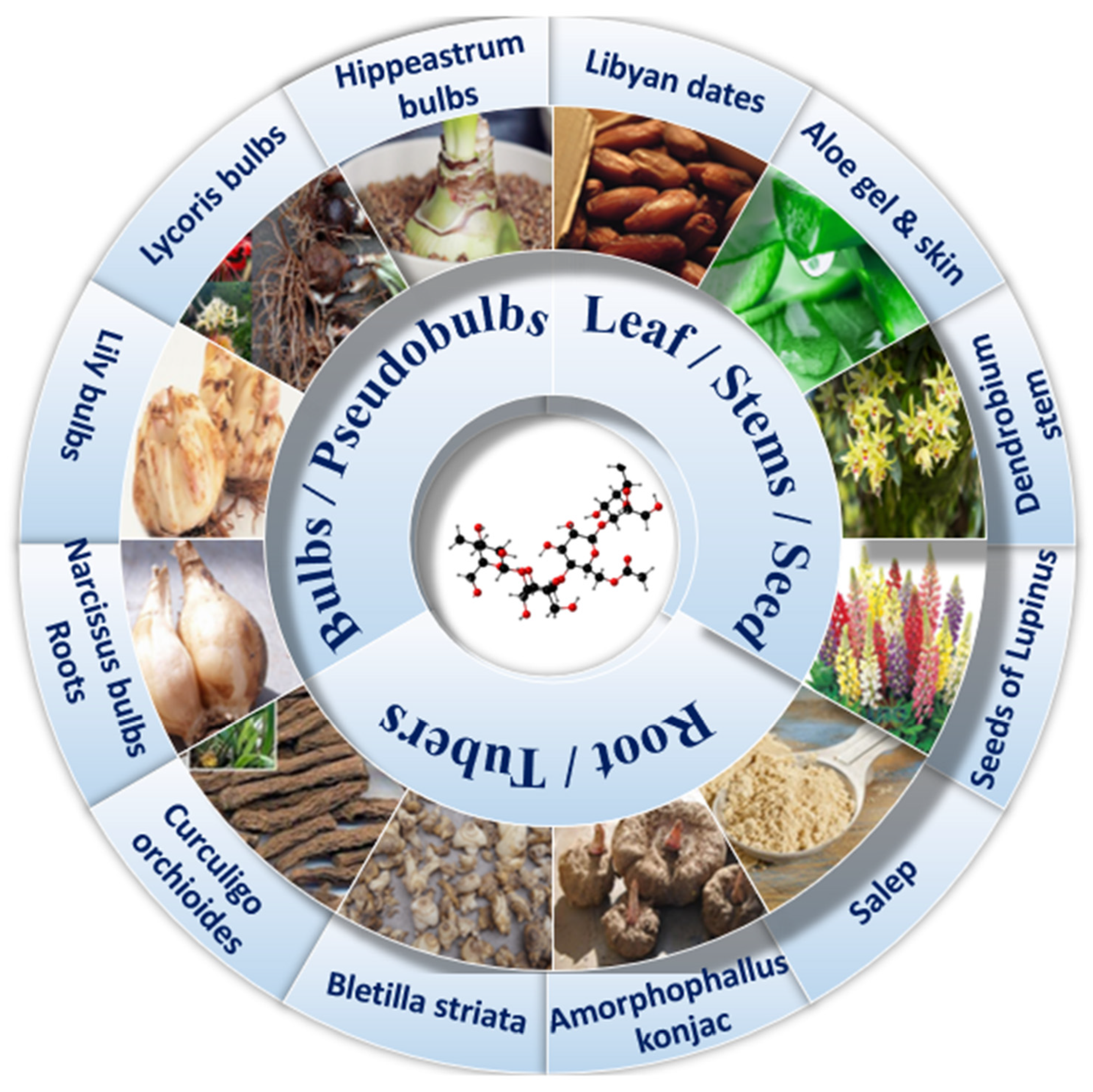
Figure 1. Glucomannan from different sources.
2. Extraction and Purification of KGM
Amorphophallus is a perennial herb of Araceae. It has up to 170 varieties, mainly distributed in Southeast Asia and Africa. These varieties are perennial plants with an underground stem in the form of a corm and a highly dissected umbrella-shaped leaf blade [22]. Konjac has a history of more than 2000 years and is a very popular food in China and Japan. Although konjac is poisonous and has a special smell, it is often called “fishy smell”. However, further processing and processing can solve this problem perfectly well. Moreover, the smaller the fishy smell, the higher the purity and the better the quality. The specific processing process is as follows.
KGM is usually obtained from natural high-quality konjac without pesticides and fertilizers (mainly 3 years old, weighing 300–1500 g), extracted by a series of broken walls and refined by multi-stage purification [23,24][23][24]. The KGM content in fresh konjac tubers is about 30%, and the highest content of refined konjac flour is 96.9% [25]. From the structure of konjac corm, KGM is mainly distributed in the storage parenchyma below the epidermis of konjac, where a large number of idioblast (also known as cystic cells) and ordinary cells are uniformly dispersed [26]. Ordinary cells contain starch, cellulose, and other components, and the texture is more brittle and very easy to break into dust. However, KGM only exists in idioblast with high hardness, good toughness, and is not easy to break [26]. Although there is a certain amount of crude protein, cellulose, and mineral elements in idioblast, the purity of KGM is enough to meet its medical and food applications.
At present, there are mainly two ways of dry processing and wet processing in industry. The steps of dry processing include washing, peeling, slicing, fixing, drying, grinding and screening of konjac (Figure 2) [27]. In principle, according to the differences in composition, toughness, and hardness between idioblast and ordinary cells, ordinary cells are broken first by mechanical crushing, in which starch, cellulose, and other impurities are gradually crushed into konjac fly powder, while idioblast will not be broken under general crushing conditions, still maintaining the integrity of the particles. Through high-intensity repeated collision and friction, the impurities on the surface of the particles will continue to detach, and then be removed by sieving or wind separation, leaving translucent konjac flour particles. Usually, the KGM treated in this way has a slight smell, but when added to food or water, the smell can also be well masked. Therefore, most people can accept it.
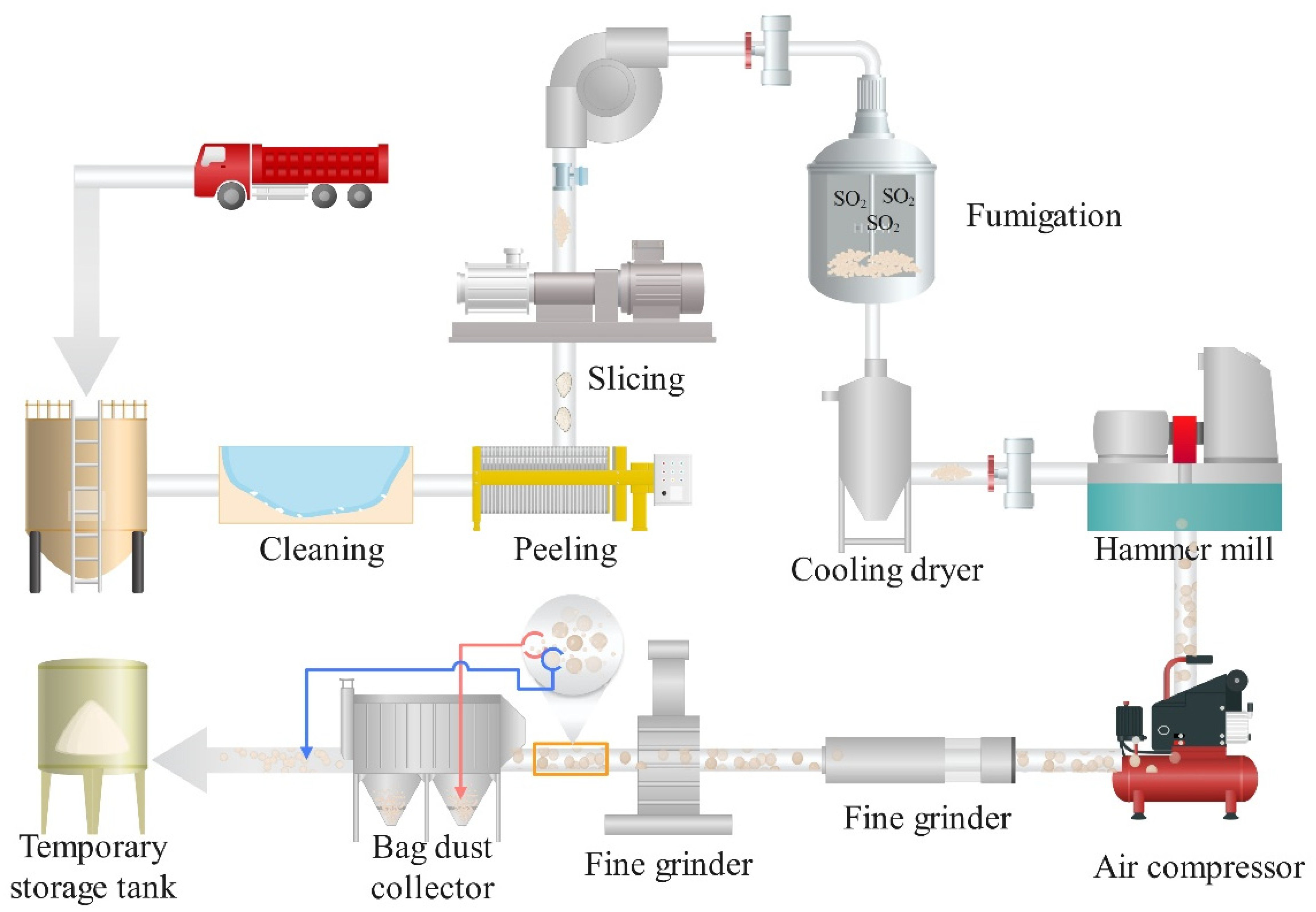
Figure 2. Flow chart of dry preparation of KGM in industry. After washing, peeling, and slicing, the konjac chips will be mixed with SO2 and hot air in a special device, and the color will be determined by fumigation, then it will be put into the drying equipment immediately, and the konjac chips will be crushed to the powder by hammer grinder and fine grinder. Finally, under the action of grinding and sorting machine and bag dust collector, the finer flying powder such as starch and cellulose will be removed, and only KGM will be retained.
The wet processing principle of KGM is similar to that of the dry process, except that the wet process uses a liquid medium when it is subjected to various mechanical forces such as shear, impact, and extrusion. Its advantage is that in the process of contact between konjac and liquid medium, the soluble impurities in idioblast will gradually dissolve out and then be removed by solid–liquid separation, leaving only glucomannan particles with higher purity. However, KGM is easy to swell and agglomerate in the presence of water, so some blocking solvents (such as ethanol and isopropanol) need to be added. For example, ethanol precipitation is the most commonly used method to obtain refined KGM in the laboratory [28]. Compared with dry processing, wet processing has the advantages of effective impurity removal, higher yield, and better viscosity. It has absolute advantages in safety and environmental protection, and is more favored by researchers. However, wet processing has seasonal requirements for konjac, and more importantly, has a high cost, which limits its application in the industrial processing of konjac flour [29,30][29][30].
3. Chemical Structure and Physicochemical Properties of KGM
3.1. Chemical Structure of KGM
The chemical structure of polysaccharides has a strong influence on their functional and nutritional properties or biological activity [31]. The basic framework of KGM is composed of D-glucose residues and D-mannose residues (molar ratio 1:1.6~1.7) and is polymerized by β-1,4-pyranoside bonds [32]. Part of the side chain can be formed at the C-3 position of the main chain mannose or the C-6 position of the sugar unit, by the linkage between acetyl groups (Figure 3) [33]. KGM has two native conformations, alpha (amorphous) and beta (crystalline) [34]. The molecular weight distribution of KGM is approximately normal since it is a relatively homogeneous polysaccharide. However, KGM has different molecular weight distributions that are affected by their origin, processing, and storage times [24]. It is reported that KGM has a mean molecular weight of 5.83 × 105 g/mol [35].
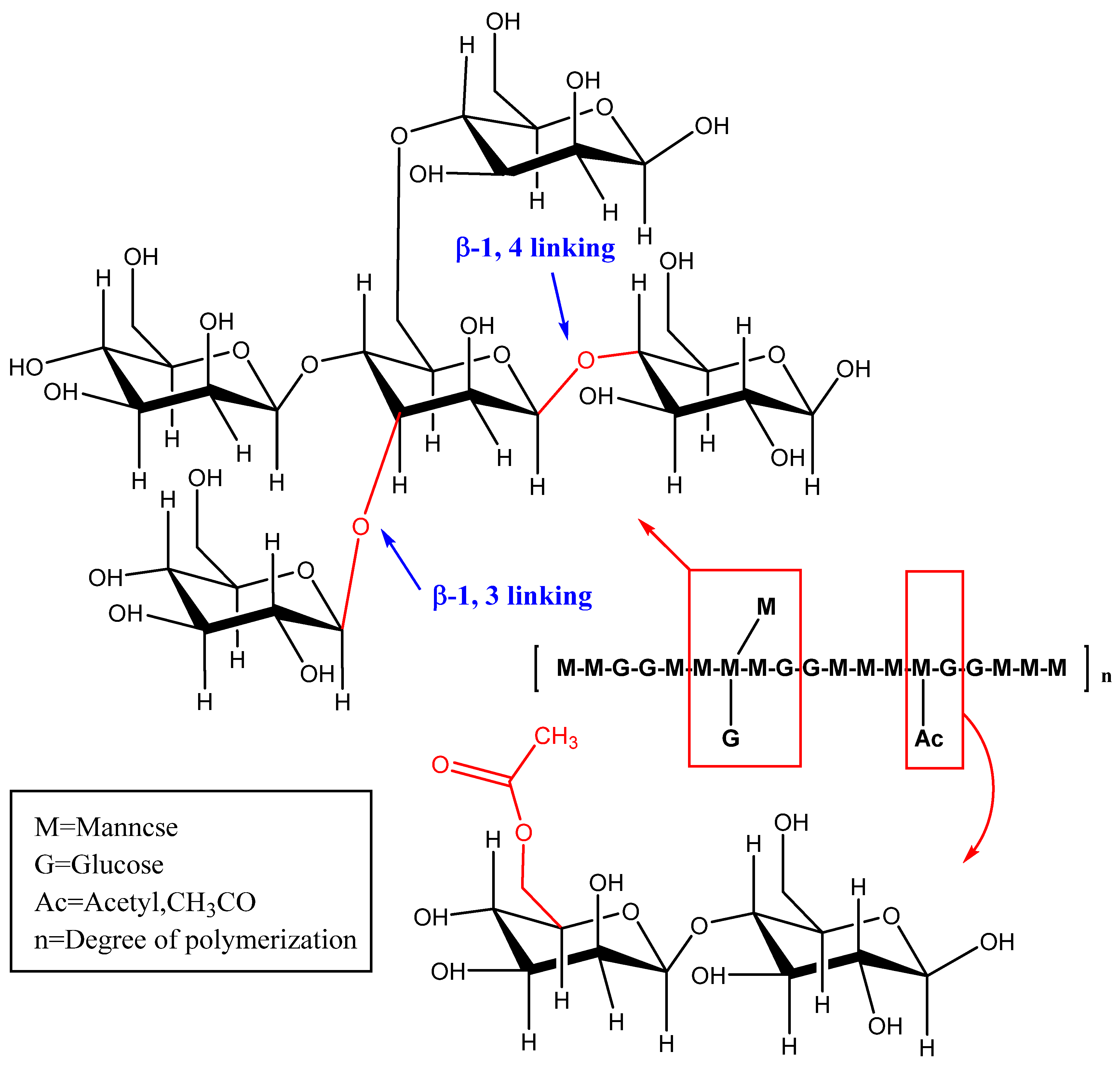
Figure 3.
Schematic diagram of KGM structure.
3.2. Physical and Chemical Properties and Function of KGM in Food and Diabetes
When KGM is dissolved in water, it can maintain high performance in an acidic environment, but it is easily unstable in an alkaline environment. For example, these gels remain essentially unchanged in strength, even after repeated heating at 100 °C [36]. A possible explanation for this may be that the acetyl group of KGM is lost under alkaline conditions, which causes aggregation and entanglement of split self-body substrates, which eventually results in the formation of a localized, continuous gel reticular structure (Figure 4) [37]. These properties may also explain the reduced diffusion of glucose in the gut, such as the complex network of gel films that prevent glucose transfer through dialysis.
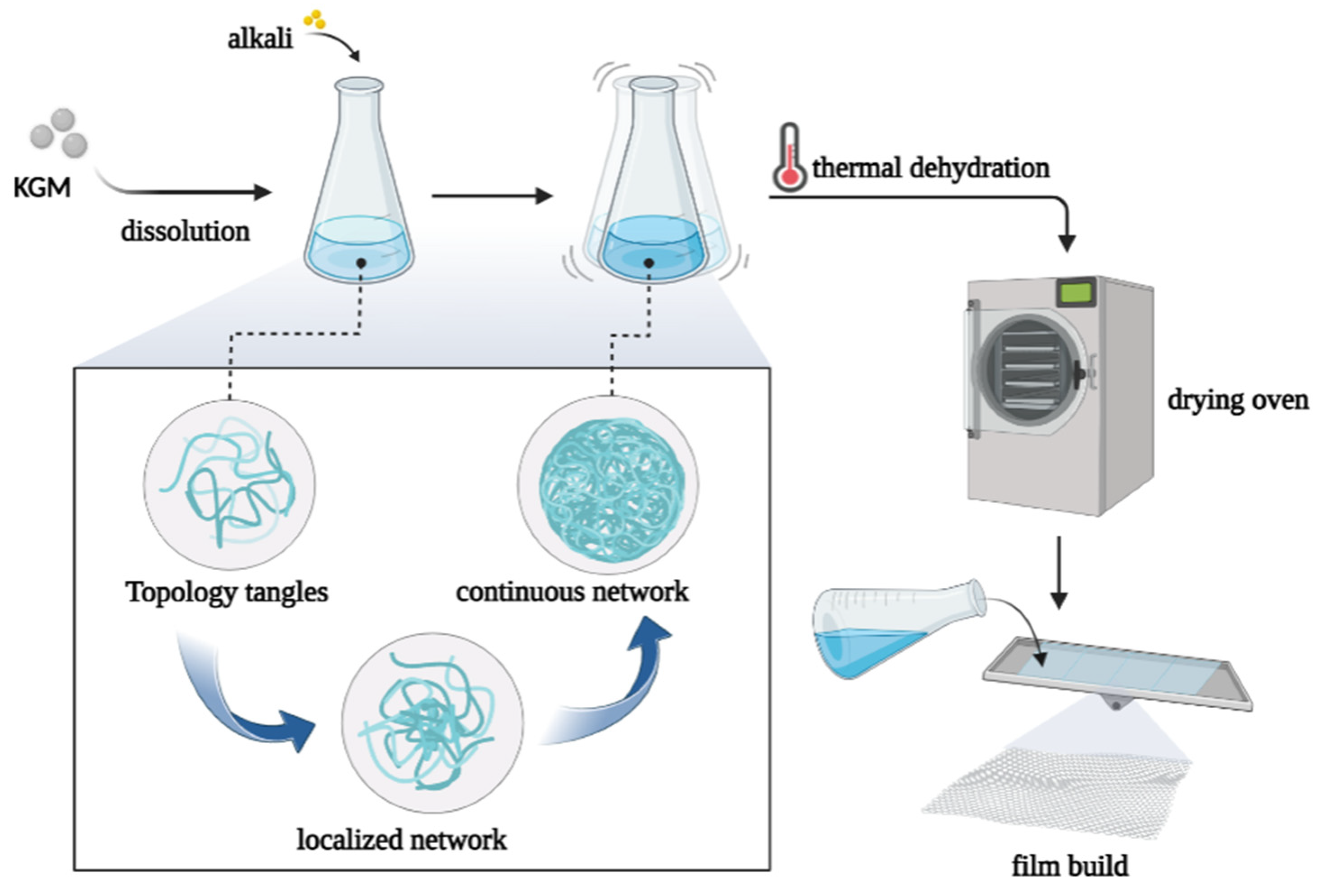
Figure 4.
Alkali treatment causes the molecules of KGM to gather and form gels, which are then heated and dehydrated to obtain thin films.
Furthermore, the role of KGM is also related to viscosity and rheological properties. Firstly, KGM can form a high viscosity solution because of its high molecular weight, strong adsorption to water, and electrical neutrality [31]. The apparent viscosity of 1% (w/w) KGM is about 30,000 cps, making it the most viscosity natural polysaccharide [38,39][38][39]. A moderate increase in dietary viscosity by KGM will help T2DM to meet the challenge of hunger in weight management. Secondly, fiber can lessen the rise in postprandial blood glucose and insulin concentration in both normal and diabetic individuals due to its rheological properties [40]. KGM solution is a typical pseudoplastic fluid with shear-thinning properties. The rheological curve can be fitted by the power law equation τ = KDn, where τ is shear stress (Pa), K is viscosity index (Pa·sn), and D is shear rate (s−1) [41]. The mechanism by which KGM improves metabolic control may be related to its rheological properties, such as increasing the viscosity of digestive juices and slowing down the absorption of food in the small intestine, thus reducing postprandial blood glucose and prolonging satiety.
The physical and chemical properties of KGM not only bring many benefits to diabetics, but they also provide more choices for the diabetic diet. Many konjac foods can be produced by using the gelling property of KGM, including konjac powder, konjac knot, konjac cake, konjac tofu, and konjac jelly. It not only meets people’s psychological and taste needs, but also maintains the effects of konjac weight loss and fullness, promotes oral health, enhances the growth and vitality of beneficial organisms in the colon, and has a combination of nutrients (such as cholesterol). KGM can also be used as a preservative and fat substitute. The diet of diabetics is affected by diseases, so they should reasonably distribute their food intake among three meals a day, KGM can be added to meat products as a fat substitute, which can effectively improve meat texture, thicken, reduce fat, and improve water retention [42]. Low-glycemic index (GI) diets are thought to reduce postprandial glycemia, resulting in more stable blood glucose concentrations [43,44][43][44]. For example, the GI of most fruits and vegetables is relatively low, so it is suitable for consumption by those with T2DM [45]. KGM can be used as a fresh-keeping agent for fruits and vegetables to give patients a better experience in vision and taste [46,47][46][47].
References
- Fletcher, B.; Gulanick, M.; Lamendola, C. Risk Factors for Type 2 Diabetes Mellitus. J. Cardiovasc. Nurs. 2002, 16, 17–23.
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 36, S67–S74.
- Griffin, S. Diabetes precision medicine: Plenty of potential, pitfalls and perils but not yet ready for prime time. Diabetologia 2022, 65, 1913–1921.
- Maskarinec, G.; Raquinio, P.; Kristal, B.; Wilkens, L.; Franke, A.; Lim, U.; Marchand, L.L.; Lampe, J.; Hullar, M. The Gut Microbiome and Diabetes Status in the Multiethnic Cohort. Curr. Dev. Nutr. 2020, 4, 1450.
- Jiang, H.; Cai, M.; Shen, B.; Wang, Q.; Zhang, T.; Zhou, X. Synbiotics and Gut Microbiota: New Perspectives in the Treatment of Type 2 Diabetes Mellitus. Foods 2022, 11, 2438.
- Sun, J.; Ren, J.; Hu, X.; Hou, Y.; Yang, Y. Therapeutic effects of Chinese herbal medicines and their extracts on diabetes. Biomed. Pharmacother. 2021, 142, 111977.
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus—Present and future perspectives. Nat. Rev. Endocrinol. 2012, 8, 228–236.
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk Factors Contributing to Type 2 Diabetes and Recent Advances in the Treatment and Prevention. Int. J. Med. Sci. 2014, 11, 1185–1200.
- Zhou, K.; Pedersen, H.K.; Dawed, A.Y.; Pearson, E.R. Pharmacogenomics in diabetes mellitus: Insights into drug action and drug discovery. Nat. Rev. Endocrinol. 2016, 12, 337–346.
- Lee, S.-H.; Yoon, K.-H. A Century of Progress in Diabetes Care with Insulin: A History of Innovations and Foundation for the Future. Diabetes Metab. J. 2021, 45, 629–640.
- Ojo, O. Dietary Intake and Type 2 Diabetes. Nutrients 2019, 11, 2177.
- Mao, T.; Huang, F.; Zhu, X.; Wei, D.; Chen, L. Effects of dietary fiber on glycemic control and insulin sensitivity in patients with type 2 diabetes: A systematic review and meta-analysis. J. Funct. Foods 2021, 82, 104500.
- Connolly, M.L.; Lovegrove, J.A.; Tuohy, K.M. Konjac glucomannan hydrolysate beneficially modulates bacterial composition and activity within the faecal microbiota. J. Funct. Foods 2010, 2, 219–224.
- Tester, R.F.; Al-Ghazzewi, F.H. Beneficial health characteristics of native and hydrolysed konjac (Amorphophallus konjac) glucomannan: Health characteristics of native and hydrolysed konjac glucomannan. J. Sci. Food Agric. 2016, 96, 3283–3291.
- Wu, X.; Song, M.; Qiu, P.; Li, F.; Wang, M.; Zheng, J.; Wang, Q.; Xu, F.; Xiao, H. A metabolite of nobiletin, 4′-demethylnobiletin and atorvastatin synergistically inhibits human colon cancer cell growth by inducing G0/G1 cell cycle arrest and apoptosis. Food Funct. 2018, 9, 87–95.
- Zhou, Y.; Qin, J.; Wang, Y.; Wang, Y.; Cheng, Y. Gastrointestinal and metabolic effects of noodles-based konjac glucomannan in rats. Food Nutr. Res. 2019, 63.
- Nagasawa, T.; Kimura, T.; Yoshida, A.; Tsunekawa, K.; Araki, O.; Ushiki, K.; Ishigaki, H.; Shoho, Y.; Suda, I.; Hiramoto, S.; et al. Konjac Glucomannan Attenuated Triglyceride Metabolism during Rice Gruel Tolerance Test. Nutrients 2021, 13, 2191.
- Yoshida, A.; Kimura, T.; Tsunekawa, K.; Araki, O.; Ushiki, K.; Ishigaki, H.; Shoho, Y.; Suda, I.; Hiramoto, S.; Murakami, M. Glucomannan Inhibits Rice Gruel-Induced Increases in Plasma Glucose and Insulin Levels. Ann. Nutr. Metab. 2020, 76, 259–267.
- Adams, J.; Hofman, K.; Moubarac, J.-C.; Thow, A.M. Public health response to ultra-processed food and drinks. BMJ 2020, 369, m2391.
- Gibney, M.J.; Forde, C.G.; Mullally, D.; Gibney, E.R. Ultra-processed foods in human health: A critical appraisal. Am. J. Clin. Nutr. 2017, 106, 717–724.
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.-C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941.
- Devaraj, R.D.; Reddy, C.K.; Xu, B. Health-promoting effects of konjac glucomannan and its practical applications: A critical review. Int. J. Biol. Macromol. 2019, 126, 273–281.
- Parry, J.-M. Konjac Glucomannan. In Food Stabilisers, Thickeners and Gelling Agents; Imeson, A., Ed.; Wiley-Blackwell: Oxford, UK, 2009; pp. 198–217. ISBN 978-1-4443-1472-4.
- Khan, H. Marya Konjac (Amorphophallus konjac). In Nonvitamin and Nonmineral Nutritional Supplements; Elsevier: Amsterdam, The Netherlands, 2019; pp. 307–312. ISBN 978-0-12-812491-8.
- Ye, S.; Zongo, A.W.-S.; Shah, B.R.; Li, J.; Li, B. Konjac Glucomannan (KGM), Deacetylated KGM (Da-KGM), and Degraded KGM Derivatives: A Special Focus on Colloidal Nutrition. J. Agric. Food Chem. 2021, 69, 12921–12932.
- Shi, Y.; Tao, Y.; Lu, Y.; Fei, X. Morphology of starch and manna granules in corms of Amorphophallus COn/ac. Guihaia 1998, 18, 75–78.
- NIE, C.; GAO, Q. Research Progress on Deep Processing and Application of Konjak. Food Sci. Technol. 2022, 47.
- Xu, W.; Wang, S.; Ye, T.; Jin, W.; Liu, J.; Lei, J.; Li, B.; Wang, C. A simple and feasible approach to purify konjac glucomannan from konjac flour—Temperature effect. Food Chem. 2014, 158, 171–176.
- Ye, T.; Wang, L.; Xu, W.; Liu, J.; Wang, Y.; Zhu, K.; Wang, S.; Li, B.; Wang, C. An approach for prominent enhancement of the quality of konjac flour: Dimethyl sulfoxide as medium. Carbohydr. Polym. 2014, 99, 173–179.
- Yuan, Z.; Wu, D.; Wu, H.; Li, X. China journal of Chinese Materia. Medica 2003, 28, 324–327.
- Tester, R.; Al-Ghazzewi, F. Glucomannans and nutrition. Food Hydrocoll. 2017, 68, 246–254.
- Cui, T.; Liu, R.; Wu, T.; Sui, W.; Zhang, M. Influence of Konjac Glucomannan and Frozen Storage on Rheological and Tensile Properties of Frozen Dough. Polymers 2019, 11, 794.
- Jiang, M.; Li, H.; Shi, J.; Xu, Z. Depolymerized konjac glucomannan: Preparation and application in health care. J. Zhejiang Univ. Sci. B 2018, 19, 505–514.
- Meng, F.; Liu, D.; LI, Y. Research progress of the structures, properties and modifications of Konjacglucomannan. Sci. Technol. Food Ind. 2016, 37, 394–400.
- Katsuraya, K.; Okuyama, K.; Hatanaka, K.; Oshima, R.; Sato, T.; Matsuzaki, K. Constitution of konjac glucomannan: Chemical analysis and 13C NMR spectroscopy. Carbohydr. Polym. 2003, 53, 183–189.
- Yang, D.; Yuan, Y.; Wang, L.; Wang, X.; Mu, R.; Pang, J.; Xiao, J.; Zheng, Y. A Review on Konjac Glucomannan Gels: Microstructure and Application. Int. J. Mol. Sci. 2017, 18, 2250.
- Hou, Y.; Nie, J. Shaoping Study and application on the deacetylated gelation of konjac glucomannan. J. Shaanxi Norm. Univ. 2022, 50, 1–14.
- Du, X.; Li, J.; Chen, J.; Li, B. Effect of degree of deacetylation on physicochemical and gelation properties of konjac glucomannan. Food Res. Int. 2012, 46, 270–278.
- Chua, M.; Baldwin, T.C.; Hocking, T.J.; Chan, K. Traditional uses and potential health benefits of Amorphophallus konjac K. Koch ex N.E.Br. J. Ethnopharmacol. 2010, 128, 268–278.
- InterAct Consortium. Dietary fibre and incidence of type 2 diabetes in eight European countries: The EPIC-InterAct Study and a meta-analysis of prospective studies. Diabetologia 2015, 58, 1394–1408.
- Yoshimura, M.; Nishinari, K. Dynamic viscoelastic study on the gelation of konjac glucomannan with different molecular weights. Food Hydrocoll. 1999, 13, 227–233.
- Chen, J.; Zhao, J.; Li, X.; Liu, Q.; Kong, B. Composite Gel Fabricated with Konjac Glucomannan and Carrageenan Could Be Used as a Cube Fat Substitute to Partially Replace Pork Fat in Harbin Dry Sausages. Foods 2021, 10, 1460.
- Vlachos, D.; Malisova, S.; Lindberg, F.A.; Karaniki, G. Glycemic Index (GI) or Glycemic Load (GL) and Dietary Interventions for Optimizing Postprandial Hyperglycemia in Patients with T2 Diabetes: A Review. Nutrients 2020, 12, 1561.
- Zafar, M.I.; Mills, K.E.; Zheng, J.; Regmi, A.; Hu, S.Q.; Gou, L.; Chen, L.-L. Low-glycemic index diets as an intervention for diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 110, 891–902.
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021, 5, 1625–1632.
- Li, X.; Xiao, N.; Xiao, G.; Bai, W.; Zhang, X.; Zhao, W. Lemon essential oil/vermiculite encapsulated in electrospun konjac glucomannan-grafted-poly (acrylic acid)/polyvinyl alcohol bacteriostatic pad: Sustained control release and its application in food preservation. Food Chem. 2021, 348, 129021.
- Hashemi, S.M.B.; Jafarpour, D. The efficacy of edible film from Konjac glucomannan and saffron petal extract to improve shelf life of fresh-cut cucumber. Food Sci. Nutr. 2020, 8, 3128–3137.
More
