Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Alexander Pavlovskii and Version 2 by Camila Xu.
Redox-active organic anode materials are endowed with obvious advantages, such as low cost, structural diversity, environmental friendliness, outstanding flexibility in their molecular design, low weight, and higher theoretical capacity, which can meet the rising demand for the energy density of next-generation battery systems. In contrast to inorganic compounds, organic materials are mainly composed of such chemical elements that are widely distributed in nature (e.g., C, H, O, N, S).
- lithium-ion batteries
- redox-active organic anode
- organic anode materials
1. Introduction
Rechargeable lithium-ion batteries (LIBs), one of the most successful commercialized secondary batteries, play a pivotal role in every aspect of our lives thanks to their suitable energy density, long service life, excellent rechargeability, and prolonged storage capacity. Nowadays, LIBs are an integral part of different consumer electronic devices, such as smartphones, laptops, watches, power banks, digital cameras, tablets, etc., and are expected to fuel plug-in hybrid electric vehicles and to be applied in smart grids in the near future. With worldwide industrial development and techno-economic growth, the energy density requirements for the above-mentioned energy-storage devices have continuously increased in the past few decades. In order to satisfy the ever-growing demand for consumer electronics and electric vehicles, achieving higher energy/power densities and longer cycle lives of LIBs has risen as a major issue for the development next generations of high-energy rechargeable Li-ion batteries.
In LIBs, the choice of electrode active materials is the dominant factor influencing the electrochemical performance of the batteries to a large extent. However, presently available commercial LIBs assembled with inorganic layered transition metal oxides, such as LiCoO2 (LCO), LiMn2O4 (LMO), LiFePO4 (LFP), LiNixCoyMn 1−x−y (NCM), etc. [1], as a cathode material and graphite or Si/C as the most commonly used anode material reach their limit in terms of sustainability, capacity, and output voltage. Graphite usually applied as a commercial anode in the current state-of-the-art LIB has poor rate capability and a low theoretical specific capacity of 372 mAh/g which significantly hampers the further development of LIBs [2][3][4][2,3,4]. Great research efforts have been put forth to find suitable alternatives to conventional graphite anodes with enhanced energy and power density. Thus, a variety of alloy-based materials (e.g., Sn, P, Ge, Si, Bi, Sb) and transition metal oxides (MxOy, M = Ni, Mn, Fe, Cr, Mo, Co, Nb, etc.) have been extensively investigated as high specific capacity LIBs anodes [5][6][7][8][9][10][11][5,6,7,8,9,10,11]. However, these anode materials for LIBs have poor electrochemical stability and fast capacity fading which impede their commercial application for Li-ion batteries.
Organic compounds with electroactive functional groups or moieties, either a polymer or small organic molecule, can undergo an electron transfer through a reversible electrochemical redox reaction. Therefore, they are suitable for the conversion and storage of chemical and electrical energy.
Compared with conventional inorganic anode materials, redox-active organic anode materials are endowed with obvious advantages, such as low cost, structural diversity, environmental friendliness, outstanding flexibility in their molecular design, low weight, and higher theoretical capacity, which can meet the rising demand for the energy density of next-generation battery systems [12][13][14][15][12,13,14,15]. In contrast to inorganic compounds, organic materials are mainly composed of such chemical elements that are widely distributed in nature (e.g., C, H, O, N, S). Thus, organic anode materials can be easily synthesized from renewable resources in facile steps [16] that have a minimal environmental footprint [17]. Moreover, both the tunable molecular structure and high structural diversity of organic compounds, unlike those of their metal-ion inorganic counterparts, allow for optimizing the electrochemical performance of organic electrode materials, such as redox activity and operating voltage in various metal-ion batteries [18][19][20][21][18,19,20,21].
For instance, the electronegativity of substituents in organic compounds could tune the redox potential. It was corroborated that the introduction of an electron-withdrawing group into the redox active organic molecule enhances the redox potential because the electron cloud around the redox center of the organic material is attracted toward higher electronegative groups, such as heteroatoms [22]. On the contrary, attaching an electron-donating group to the redox-active organic molecule reduces the redox potential [23]. It should be emphasized that some organic compounds enable the batteries to be exploited in extreme conditions, such as a high pH value, an extended temperature range from −70 to 150 °C, and the presence of oxygen [24]. With these advantages of organic electrode materials, LIBs based on organic electrodes have attracted considerable research interest and have made magnificent progress [20][24][25][26][27][28][29][20,24,25,26,27,28,29].
Due to the versatile properties of organic electrodes, various types of organic materials have been used in all kinds of rechargeable batteries, comprising all-solid-state batteries, nonaqueous sodium-ion, lithium-ion, potassium-ion, dual-ion batteries, multivalent-metal batteries, redox flow batteries, and aqueous batteries [24]. Furthermore, apart from small organic molecules, different polymers, polymer frameworks, and 2D organic materials can also be adopted as organic electrodes in rechargeable batteries [30][31][32][33][30,31,32,33].
For a long time, organic anode materials have received much less attention compared to their inorganic counterparts mostly because of their relatively low electronic conductivity and the tremendous success of inorganic anode materials in either application or research. Although there are already numerous reviews of organic electrode materials used in different kinds of rechargeable batteries [14][16][17][24][25][34][35][36][37][14,16,17,24,25,34,35,36,37], a review article that briefly, simply, and systematically introduces the fundamental knowledge of organic anode materials for LIBs is still absent.
2. Fundamentals of Organic Anode Materials
2.1. Basic Components of an Electrochemical Cell
Rechargeable (also called “secondary”) batteries consist of two electrodes, a cathode (or positive electrode) with high redox potential and an anode (or negative electrode) with lower redox potential, in contact with an electrolyte (either solid or liquid). The electrolyte system with low viscosity, high stability, and ionic conductivity at a large potential window is preferred. Furthermore, electrolytes must be inert towards both the cathode and active anode materials. The cathode and anode of the cell are connected through an external circuit. To avoid short circuits, the cathode is mechanically separated from the anode by an electronically insulating porous membrane (usually named separator). These are the widespread cell components for a battery. Figure 1 depicts a schematic of a typical battery design.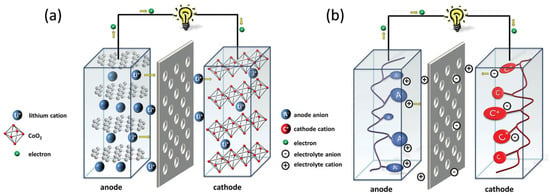
Figure 1. Schematic showing of the processes occurring during discharging of (a) traditional inorganic and (b) organic electrode-based Li-ion batteries. Instead of intercalation-based reactions in inorganic compounds, organic compounds undergo surface-based redox reactions. Reprinted with permission from Ref. [38], Copyright 2016, American Chemical Society.
2.2. Mechanism of Charge Storage in Lithium-Ion Batteries and Working Principles of Organic Anode Materials
During the charging process of a battery, electrons are forced (by an applied potential or current) from the positive electrode to the negative electrode, oxidizing the cathode and reducing the anode. During the discharge process of a battery, electrons spontaneously flow in the opposite direction (from the negative electrode to the positive electrode), reversing the previous redox reactions. Thus, both positive and negative electrodes, with various redox potentials, must be capable of reversible electrochemical redox reactions. The difference in chemical potential between these reactions constitutes the operating voltage of the cell, also known as the open circuit voltage. In contrast to inorganic electrode materials, with redox reactions that are based on the valence change of the elemental substance or transition metal, the redox reaction of organic electrode materials is based on the change in the charge state of the redox active organic species or an electroactive organic group. When an active electrode material changes its state of charge, oppositely charged ions in the electrolyte diffuse into the active material to charge compensate. Depending upon the potential of this redox process, organic materials can either be suited for use as a cathode or anode active material. The polarized state of organic active material is used for interaction with the mobile ion. In other words, a reversible metal ion intercalation-based electrochemical redox reaction is employed to store the charge in conventional inorganic-based electrode materials. In organic electrode materials, a change in the charge state of the redox active moieties is utilized to store the charge. During the charge and discharge electrochemical process, the inorganic-based electrode structure is stabilized by the change in the oxidation states of the transition metals from inorganic cathode materials for LIBs, while in organic electrode material, the polarized state of organic active material is commonly balanced by its interaction with metal ions from the electrolyte.2.3. Types of Organic Anode Materials and Their Advantages
According to different redox reactions of organic electrode materials, they can be generally classified as N-type, P-type, or bipolar (Figure 2). N-type organics obtain a negative charge (N−) from the original neutral state (N) during the electrochemical reduction reaction and revert to their initial state by oxidation. P-type organic compounds (P) are oxidized during the redox process yielding positively charged cations. B-type (or bipolar) organics are another type of organic compound, for which the initial neutral state (B) can be either oxidized to a positively charged state (B+) or reduced to a negatively charged state (B−), which depends on the applied voltage. B-type organic compounds can be also considered N- or P-type organics since, in general, only half of the reaction takes place on one electrode.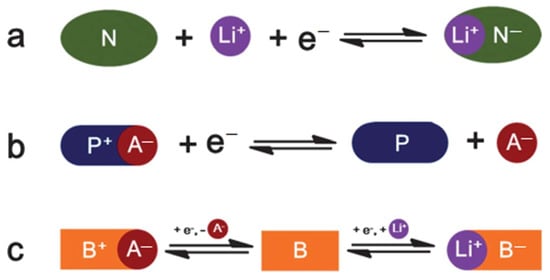
Figure 2. The reversible redox reaction of three types of redox-active organic compounds: (a) N-type; (b) P-type; (c) bipolar. The anion of the electrolyte is denoted as A−. Used with permission from the Royal Society of Chemistry, from Ref. [39]; permission conveyed through Copyright Clearance Center, Inc.
2.4. Advantages of Organic Anode Materials
It should be noted that in traditional inorganic intercalation-based electrode materials, lithium-ion insertion leads to the transformation of the cathode lattice structure. This result in heat generation and slow reaction kinetics during the charge/discharge process. At the same time, fast insertion and de-insertion of lithium-ion may result in the structural degradation of the cathode material. These challenges substantially affect both the rate and cyclic performance of the typical LIBs, impeding their role in long-life and high-power applications. In contrast, organic electrode materials undergo fast and simple redox reactions without structural degradation of the electrode active material. Thus, they exhibit excellent cyclic and rate performance. Moreover, as previously stated, inorganic intercalation-based materials are very sensitive to the ionic radius of cation, whereas, in organic electrode materials, lithium cation can be easily substituted with various alkali metal cations with a bigger ion radius, such as Na+, K+, etc., without any significant fade of the capacity of batteries. In addition, both the larger interlayer spacings and greater flexible structures of organic electrode materials (Figure 3) afford faster ion diffusion and reduced structural and volumetric changes upon charge/discharge processes, enhancing rate capabilities [25].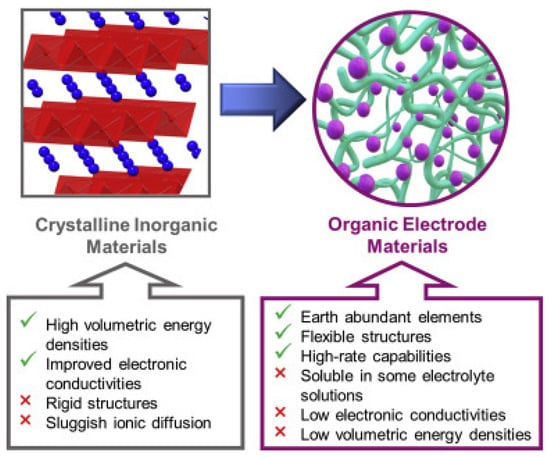
Figure 3. Schematic illustration of the unique properties of organic electrode materials which make them particularly attractive for use as high-performance electrode materials in rechargeable LIBs [25]. Ref. [25] is an open-access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
