Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Alan Prem Kumar and Version 2 by Conner Chen.
Cucurbitacins constitute a group of cucumber-derived dietary lipids, highly oxidized tetracyclic triterpenoids, with potential medical uses. These compounds are known to interact with a variety of recognized cellular targets to impede the growth of cancer cells.
- cucurbitacins
- anti-proliferation
- apoptotic
1. Apoptotic and Cell-Cycle Arrest
Apoptotic cell death can be triggered in cancer through internal and extrinsic processes, which converge on the control of caspase-dependent proteolysis of cellular proteins and DNA fragmentation [1][2][3][30,31,32]. Similarly, all tumor types have abnormal cell-cycle progression activity, which acts as a catalyst for carcinogenesis [4][33]. Recent research has shown that a variety of biological processes are regulated by cell-cycle proteins [5][6][7][34,35,36]. Therefore, numerous chemo-preventive FDA-drugs have been shown to mediate antitumor effects either via activation of apoptotic or cell-cycle arrest (Figure 12) signaling pathways [8][9][10][37,38,39]. For instance, results from Li et al. (2018) revealed that cucurbitacin I caused lung cancer (A549) cells to undergo excessive ERS, CHOP-Bax and caspase-12-dependent ERS-associated apoptosis [11][40]. In colorectal cancer (SW480 and Caco-2) cells, treatment with cucurbitacin B resulted in cell-cycle arrest at the G1 phase as well as decreased Cyclin D1 and Cyclin E1 levels. Both CRC cell lines underwent in vitro cell death when exposed to cucurbitacin B (CuB), which was accompanied by caspase-3 and cleaved PARP [12][41]. Using triple negative breast cancer (TNBC), cucurbitacin E strongly boosted JNK activation while considerably decreasing AKT and ERK activation in MDA-MB-468 cells. It also significantly decreased expression of Cyclin D1, Survivin, XIAP, Bcl2 and Mcl-1 [13][42]. In the pancreatic cancer cell line Capan-1, CuD induced cell-cycle arrest and death via the ROS/p38 pathway [14][43]. Cucurbitacin I-induced cell death in ovarian cancer (SKOV3) included apoptosis, as evidenced by upregulated caspase 3 and BAX and a decrease in Bcl2 [15][21]. Flow cytometric measurement of DNA content and RT-PCR analyses suggested that cucurbitacin B caused G2/M arrest in human breast cancer cell lines (MDA-MB-231 and MCF-7) through elevated p21 expression [16][44]. Huang et al. showed that in human bladder cancer (T24) cells, cucurbitacin E-induced G2/M arrest was accompanied by a significant rise in p53 and p21 levels and a fall in the levels of STAT3, cyclin-dependent kinase 1 (CDK1) and cyclin B [17][45]. In addition, cucurbitacin E-induced G2/M phase arrest and death of T24 cells also depended on Fas/CD95 and mitochondria-dependent apoptotic pathways. Similarly, using other cancerous cell lines, cucurbitacins target the cell-cycle actions that involves growth inhibition, cell-cycle arrest at G2/M phase and induction of apoptosis [18][46]. Cucurbitacin I has been observed to suppress phosphotyrosine STAT3 in human cancerous lung cells [19][47]. Recently, it was observed to promote gastric cancer cell apoptosis by inducing the production of cellular ROS, as well as the endoplasmic reticulum stress pathway [11][20][40,48]. While cucurbitacin B, E and I have been observed to inhibit both JAK2 and STAT3 activation, cucurbitacin A and I have been reported to inhibit JAK2 and STAT3, respectively [19][47]. Treating Hep-2 cells with different concentrations of cucurbitacin B for various time intervals showed reduction in cell proliferation, cell-cycle distribution, and increased cell apoptosis in cancerous cell lines [18][46]. This study also stated that cucurbitacin B exhibited significant efficacy in inhibiting cell growth, arresting cell cycle at the G2/M phase, and inducing apoptosis in a dose- and time-dependent manner [18][46]. Similarly, cucurbitacins B, D, E were observed to inhibit proteins such as JAK-STAT3. They also inhibited mitogen-activated protein kinases (MAPK)- signaling pathways and tumor angiogenesis [20][48]. A study conducted on human umbilical vascular endothelial cell lines revealed cucurbitacin to significantly inhibit the proliferation, migration, and angiogenesis. It also blocked essential proteins such as Jak2-signal transducer, vascular endothelial growth factor receptor (VEGFR) and STAT3 signaling pathways [21][49]. Such studies have highlighted that the main mechanism involved in imparting the anti-tumorigenic potentials of cucurbitacins involves inhibition of the JAK/STAT3 signaling pathway, which plays an essential role in activation, proliferation, and maintenance of cancerous cells [22][14]. Another recent study has shown that treatment with 8 µM cucurbitacin IIb for 24 h remarkably inhibited the proliferation of HeLa and A549 tumor cells, with IC50 values of 7.3 and 7.8 µM, respectively, while increasing total apoptosis by 56.9 and 52.3%, respectively [23][50]. Another pathway by which cucurbitacin IIb induces apoptosis and cell-cycle arrest is by the regulating EGFR/MAPK pathway [24][51]. Similarly, cucurbitacin D was observed to regulate the levels of oncogenic signaling cascades, JAK/STAT, Wnt/β-catenin and associated non-coding RNAs in many cancer cell lines [25][52]. Recent studies have shown that CuIIb and cucurbitacin B induced apoptosis in cervical cancer cell lines by Nrf2 inhibition, whereas in lung cancer cell lines cucurbitacin B was responsible for suppressing growth and inducing apoptotic death by impeding IL-6/STAT3 signaling [15][26][21,53].
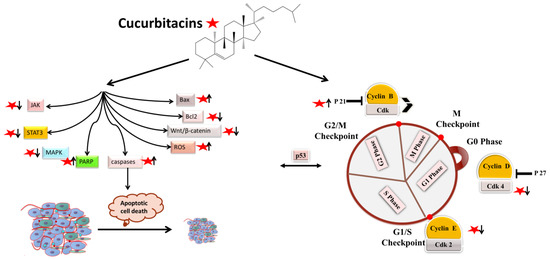
Figure 12.
Molecular targets of cucurbitacins in modulating cell-cycle progression and inducing apoptotic cell death.
2. Antiangiogenic and Antimetastatic Mechanisms
The physiological process by which new blood vessels develop from pre-existing vessels is known as angiogenesis. Anti-angiogenesis causes suppression of tumor growth because of hunger and toxic waste buildup in its microenvironment [27][28][54,55]. The development and metastasis of the tumor have a major impact on the cancer vasculature (Figure 23). Vascular endothelial growth factors (VEGFs) are crucial protein regulators of angiogenesis and metastasis. Studies have shown that inhibiting the VEGFR2-mediated JAK/STAT3 pathway is considered as an effective approach to suppress angiogenesis [21][49]. Though many studies about the mechanism of cucurbitacins and angiogenesis are not well known, few studies have still shown that cucurbitacins such as cucurbitacin B, cucurbitacin D, cucurbitacin E and cucurbitacin I possess anti-angiogenesis properties [29][30][56,57]. CuB significantly inhibited angiogenesis, metastasis, and vascular development in dose-dependent manner in in vivo models and chick embryos [29][56]. CuE significantly inhibited human umbilical vascular endothelial cell (HUVEC) proliferation and angiogenesis by targeting the VEGFR2-mediated Jak2/STAT3 signaling pathway [21][49]. CuB has been observed to inhibit ERK1/2, prevent Raf-MEK-ERK from activating STAT3, which ultimately plays a key role in angiogenesis [31][58]. A similar effect of CuB was seen also in human breast cancer cell lines. It successfully inhibited angiogenesis by targeting the FAK/MMP-9 signaling axis [32][59]. CuB showed antimetastatic activity and targeted angiogenesis also in paclitaxel resistant A2780/Taxol ovarian cancer cells. It also suppressed angiogenesis by downregulating the expression of HIF-1 targets, VEGF, VEGFR2 phosphorylation and erythropoietin [29][33][56,60]. Another study revealed the effective use of CuE for anti-angiogenesis in Huh7 cells. It decreased the tube formation in HUVECs and was also responsible for inhibiting the process of neo-vascularization in CAM assays [34][61]. A recent study showed that CuE modulated the JAK/STAT3 pathways, which regulated the angiogenesis [35][62]. CuE has been also involved in inhibiting the KDR/VEGFR2-mediated pathway of angiogenesis [36][63]. Treating A549 cells with cucurbitacins for ~21 days showed positive results for inhibiting metastasis by regulating the levels of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D11 [37][64]. Similarly, other cucurbitacins were observed to inhibit angiogenesis in MDA-MB-231 and MCF-7 cancer cells by inhibiting the JAK/STAT pathways [38][65].
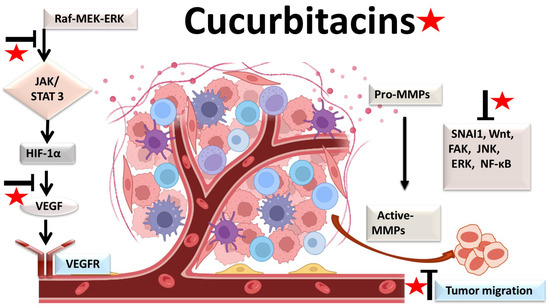
Figure 23.
Molecular targets of cucurbitacins in suppressing angiogenesis and impeding cancer cell metastasis.
3. Anti-Inflammatory Mechanisms
Most malignancies’ growth and malignant progression are correlated with inflammation [39][40][41][66,67,68]. Both intrinsic and extrinsic inflammations have the potential to inhibit the immune system, which creates an ideal environment for the growth of tumors [42][43][44][69,70,71]. As a result, focusing on inflammation is a tempting strategy for both cancer therapy and cancer prevention [42][45][69,72]. Cucurbitacins have been observed to interact with proteins associated with inflammatory (Figure 34) pathways such as interleukins (IL)-6, IL-5, IL-1β, IL-12, IL-13 in a dose-dependent manner [46][73]. Dietary cucurbitacin E has been shown to reduce inflammation and immunosuppression by downregulating the NF-κB signaling pathway [47][74]. CuB has been studied to possess protective effects by reducing inflammatory responses on sepsis-induced acute lung injury in in vivo rat models. It significantly reduced the levels of TNF-α, IL-6, cytokine secretion and accumulation of inflammatory cells. It also regulated the levels of Ca2+, which play an essential role in inflammatory responses [48][75]. CuB inhibited inflammatory responses through targeting the SIRT1/IGFBPrP1/TGF β1 axis. It downregulated the expression levels of TGF β1, IGFBPrP1, and upregulated the expression of SIRT 1 [49][76]. Similarly, CuE decreased the levels of pro-inflammatory cytokines, such as IL-17 and IFN-γ, as well as the activities of the STAT3 and IL-17A-promoter in allo-reactive T cells [50][77]. CuE has been shown to inhibit skin inflammation and fibrosis by regulating the expression of α-Sma and Col-I in mice models [49][76]. Recently, it has also been demonstrated that CuE ameliorated lipopolysaccharide-evoked injuries and inflammation in bronchial epithelial cells by regulating the TLR4-NF-κB signaling. It was responsible for suppressing levels of inflammatory cytokine production, TNF-α, IL-6 and IL-8 [51][78]. Cucurbitacin B was observed to directly bind to toll-like receptor 4 (TLR4) and activate NLRP3 inflammasome, which further ultimately executed pyroptosis in A549 cells. CuB treatment has been observed to upregulate the protein expressions of IL-1β, GSDMD, HMGB1 and led to inhibition of generation of mitochondrial ROS and pyroptosis [52][79]. CuB was reported to sensitize CD133+ HepG2 cells in in vitro and in vivo models [53][80].
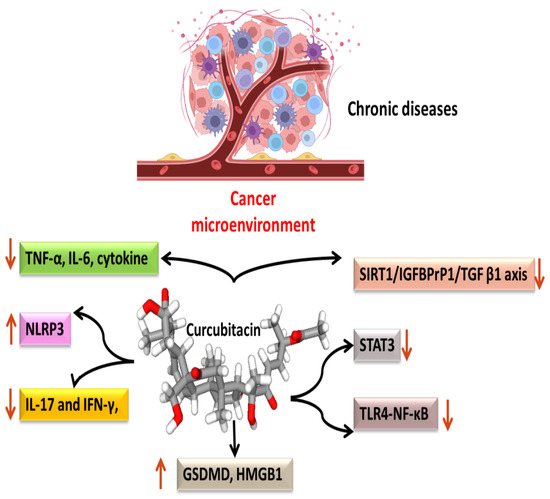
Figure 34.
Anti-inflammatory targets of cucurbitacins in malignant cells.
