The purification of biomolecules with a high degree of specificity, such as lectins, has garnered interest in the use of fixed non-traditional beds functionalized with ligands of particular interest. The interaction is both robust enough to permit the adsorption of glycoproteins and reversible enough to permit the dissociation of molecules in response to changes in the solution’s pH. Studies on unconventional adsorbents, such as chromatographic supports, can substantiate, enrich, and assist projects in various areas of knowledge. Polyacrylamide cryogens are emerging and efficient, and can be synthesized and have their matrices modified for multiple purposes and chromatographic techniques. They are also functional and have low costs compared to conventional chromatographic adsorbents. In this context, lectins can mainly be used in the prevention of autoimmune diseases and in studies with biosensors.
- purification of bio compounds
- macromolecules
- affinity chromatography
1. Introduction
2. Lectin Activity
Conceptually, lectins are proteins of non-immune origin that recognize and are associated with carbohydrates or glycoconjugates reversibly, with high affinity and specificity. Due to this ability, these biomolecules have important biological effects, such as insecticide, bactericidal, antitumor, and fungicide, in addition to an injunction on HIV-I protease, and became essential instruments in the diagnosis of diseases, identification of microorganism strains, and in studies related to blood types. Plant lectins have been used in cell biology and immunology as diagnostic and immunomodulatory agents, as well as for therapeutic purposes [16]. In addition, they can be used in the production of biosensors for the food industry, verifying the presence of microorganisms to ensure the quality of raw materials and industrialized products [17,18][17][18]. It is worth noting that the study of Matoba et al. [19] increased the antiviral activity of lectins. Such proteins have as characteristics the recognition and maintenance of specific and reversible bonds to mono- or oligosaccharides and other substances containing sugars, maintaining the covalent structure of these glycosidic ligands [19]. They can precipitate cells, glycoconjugates, and polysaccharides from animal, plant, virus, and bacterial sources [20,21][20][21]. The binding of lectins with sugars is attributed to a carbohydrate recognition domain (CRD) within their polypeptide structure. The interaction of lectins with certain carbohydrates can be as specific as the interaction between antigen and antibody or substrate and enzyme. Some are metalloproteins, in other words, they require the presence of metal cations at their specific binding sites with carbohydrates in connection with them, resembling metalloproteases; but lectins do not present catalytic activity [20,22][20][22]. Generally, lectins have at least two binding sites for carbohydrates, which allow cross-linking between cells through combinations with sugars on the surface or between sugars contained in macromolecules, justifying their ability to agglutinate particles and precipitate glycoconjugates. The lectin–carbohydrate interaction is due to covalent bonds, in which water molecules, associated with the polar group of proteins and also around the carbohydrate, are displaced (Figure 1). This modification results in the formation of new hydrogen bonding networks, which, together with van der Waals forces, stabilize this interaction [7,23][7][23].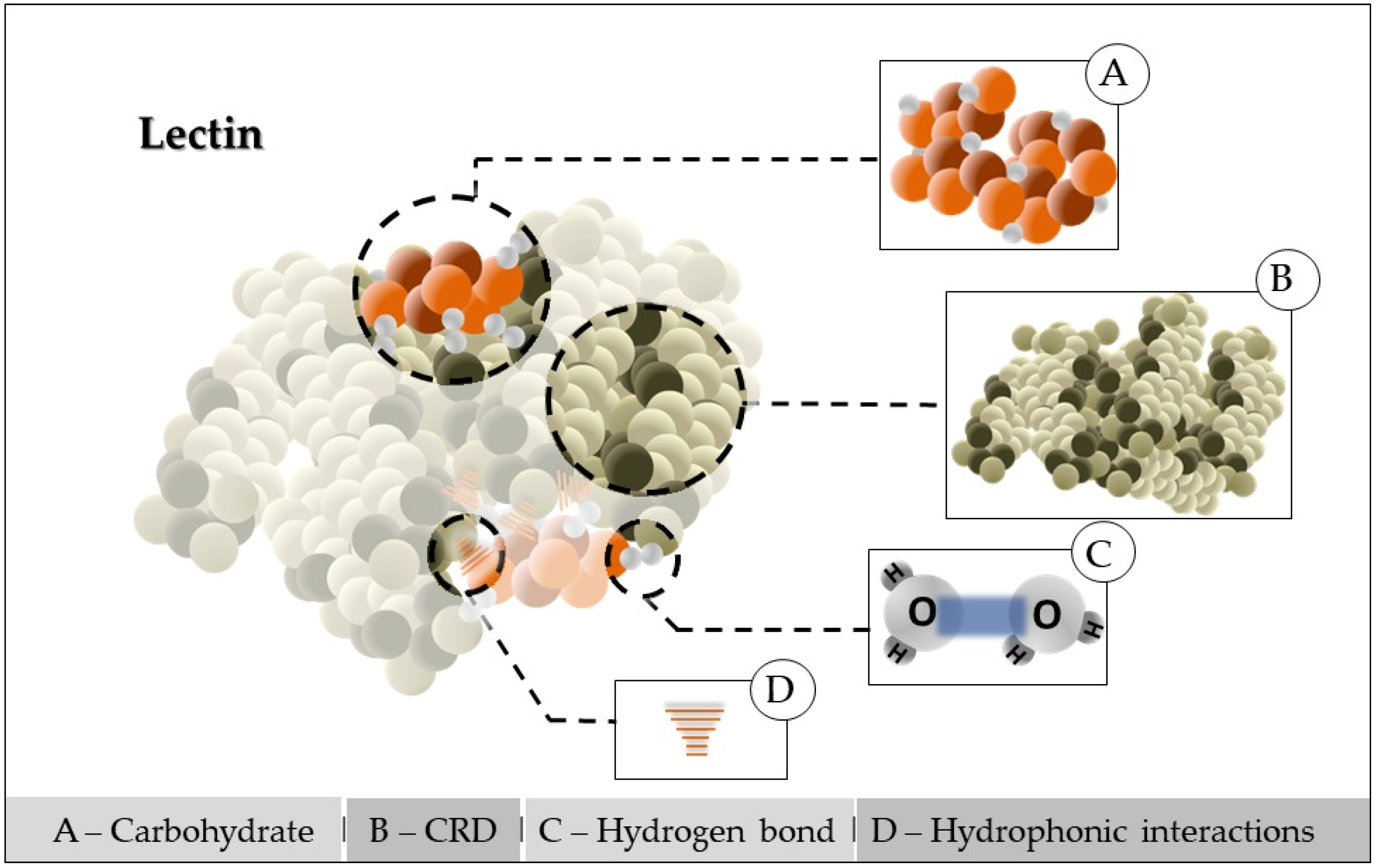
References
- Janson, J.C. Principles, High Resolution Methods, and Applications. In Protein Purification; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 3–50. ISBN 978-0-471-74661-4.
- Dainiak, M.B.; Allan, I.U.; Savina, I.N.; Cornelio, L.; James, E.S.; James, S.L.; Mikhalovsky, S.v.; Jungvid, H.; Galaev, I.Y. Gelatin-Fibrinogen Cryogel Dermal Matrices for Wound Repair: Preparation, Optimisation and in Vitro Study. Biomaterials 2010, 31, 67–76.
- Perçin, I.; Khalaf, R.; Brand, B.; Morbidelli, M.; Gezici, O. Strong Cation-Exchange Chromatography of Proteins on a Sulfoalkylated Monolithic Cryogel. J. Chromatogr. A 2015, 1386, 13–21.
- Tao, S.P.; Wang, C.; Sun, Y. Coating of Nanoparticles on Cryogel Surface and Subsequent Double-Modification for Enhanced Ion-Exchange Capacity of Protein. J. Chromatogr. A 2014, 1359, 76–83.
- Ünlüer, Ö.B.; Ersöz, A.; Denizli, A.; Demirel, R.; Say, R. Separation and Purification of Hyaluronic Acid by Embedded Glucuronic Acid Imprinted Polymers into Cryogel. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 934, 46–52.
- Banerjee, S.; Chaki, S.; Bhowal, J.; Chatterjee, B.P. Mucin Binding Mitogenic Lectin from Freshwater Indian Gastropod Belamyia Bengalensis: Purification and Molecular Characterization. Arch. Biochem. Biophys. 2004, 421, 125–134.
- Oliveira, J.T.A.; Melo, V.M.M.; Câmara, M.F.L.; Vasconcelos, I.M.; Beltramini, L.M.; Machado, O.L.T.; Gomes, V.M.; Pereira, S.P.; Fernandes, C.F.; Nunes, E.P.; et al. Purification and Physicochemical Characterization of a Cotyledonary Lectin from Luetzelburgia Auriculata. Phytochemistry 2002, 61, 301–310.
- Roy, I.; Sardar, M.; Gupta, M.N. Cross-Linked Alginate-Guar Gum Beads as Fluidized Bed Affinity Media for Purification of Jacalin. Biochem. Eng. J. 2005, 23, 193–198.
- Gonçalves, G.R.F.; Gandolfi, O.R.R.; Santos, C.M.S.; Bonomo, R.C.F.; Veloso, C.M.; Fontan, R.d.C.I. Development of Supermacroporous Monolithic Adsorbents for Purifying Lectins by Affinity with Sugars. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1033–1034, 406–412.
- Ourth, D.D.; Rose, W.M. Purification, Characterization and Seasonal Variation of Mannose-Binding C-Type Lectin in Ictalurid Catfish. Aquaculture 2011, 321, 191–196.
- Jung, W.K.; Park, P.J.; Kim, S.K. Purification and Characterization of a New Lectin from the Hard Roe of Skipjack Tuna, Katsuwonus Pelamis. Int. J. Biochem. Cell Biol. 2003, 35, 255–265.
- Arora, S.; Saxena, V.; Ayyar, B.V. Affinity Chromatography: A Versatile Technique for Antibody Purification. Methods 2017, 116, 84–94.
- Mallik, R.; Hage, D.S. Affinity Monolith Chromatography. J. Sep. Sci. 2006, 29, 1686–1704.
- Pfaunmiller, E.L.; Paulemond, M.L.; Dupper, C.M.; Hage, D.S. Affinity Monolith Chromatography: A Review of Principles and Recent Analytical Applications. Anal. Bioanal. Chem. 2013, 405, 2133–2145.
- Ferreira da Silva, J.; Lemos da Silva, D.; Gomes Nascimento, R.; Ayra Alcântara Veríssimo, L.; Martins Veloso, C.; Ferreira Bonomo, R.C.; da Costa Ilhéu Fontan, R. Enhancements in Sugar Immobilization in Polymeric Macroporous Matrices for Affinity Capture. J. Appl. Polym. Sci. 2019, 136, 47956.
- Gondim, A.C.S.; Romero-Canelón, I.; Sousa, E.H.S.; Blindauer, C.A.; Butler, J.S.; Romero, M.J.; Sanchez-Cano, C.; Sousa, B.L.; Chaves, R.P.; Nagano, C.S.; et al. The Potent Anti-Cancer Activity of Dioclea Lasiocarpa Lectin. J. Inorg. Biochem. 2017, 175, 179–189.
- Suzuki, T.; Abe, T.; Umehara, K.; Choi, J.H.; Hirai, H.; Dohra, H.; Kawagishi, H. Purification and Characterization of a Lectin from the Mushroom Hypsizigus Marmoreus. Mycoscience 2015, 56, 359–363.
- Selvaprakash, K.; Chen, Y.C. Functionalized Gold Nanoparticles as Affinity Nanoprobes for Multiple Lectins. Colloids Surf. B Biointerfaces 2018, 162, 60–68.
- Matoba, Y.; Sato, Y.; Oda, K.; Hatori, Y.; Morimoto, K. Lectins Engineered to Favor a Glycan-Binding Conformation Have Enhanced Antiviral Activity. J. Biol. Chem. 2021, 296.
- Gajbhiye, V.; Gong, S. Lectin Functionalized Nanocarriers for Gene Delivery. Biotechnol. Adv. 2013, 31, 552–562.
- He, S.; Shi, J.; Walid, E.; Zhang, H.; Ma, Y.; Xue, S.J. Reverse Micellar Extraction of Lectin from Black Turtle Bean (Phaseolus Vulgaris): Optimisation of Extraction Conditions by Response Surface Methodology. Food Chem. 2015, 166, 93–100.
- Lavín de Juan, L.; García Recio, V.; Jiménez López, P.; Girbés Juan, T.; Cordoba-Diaz, M.; Cordoba-Diaz, D. Pharmaceutical Applications of Lectins. J. Drug Deliv. Sci. Technol. 2017, 42, 126–133.
- Santos, A.L.E.; Júnior, C.P.S.; Neto, R.N.M.; Santos, M.H.C.; Santos, V.F.; Rocha, B.A.M.; Sousa, E.M.; Carvalho, R.C.; Menezes, I.R.A.; Oliveira, M.R.C.; et al. Machaerium Acutifolium Lectin Inhibits Inflammatory Responses through Cytokine Modulation. Proc. Biochem. 2020, 97, 149–157.
- Carrillo, C.; Cordoba-Diaz, D.; Cordoba-Diaz, M.; Girbés, T.; Jiménez, P. Effects of Temperature, PH and Sugar Binding on the Structures of Lectins Ebulin f and SELfd. Food Chem. 2017, 220, 324–330.
- Singh, A.; Trans, K.S.-C.S. Effect of Temperature, PH and Denaturing Agents on Biological Activity of MCJ Lectin. Chem. Sci. Trans. 2013, 2.
- Sharon, N.; Lis, H. Legume Lectins—A Large Family of Homologous Proteins. FASEB J. 1990, 4, 3198–3208.
- Kanellopoulos, P.N.; Tucker, P.A.; Pavlou, K.; Agianian, B.; Hamodrakas, S.J. A Triclinic Crystal Form of the Lectin Concanavalin A. J. Struct. Biol. 1996, 117, 16–23.
