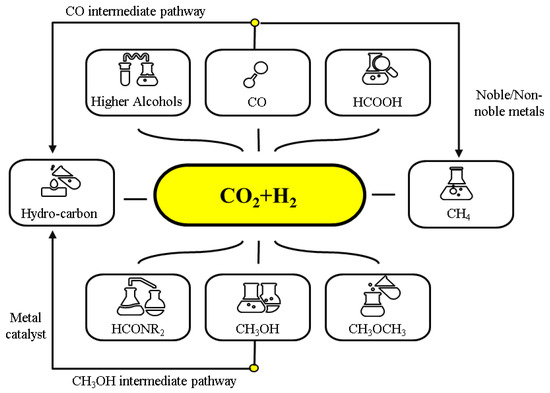
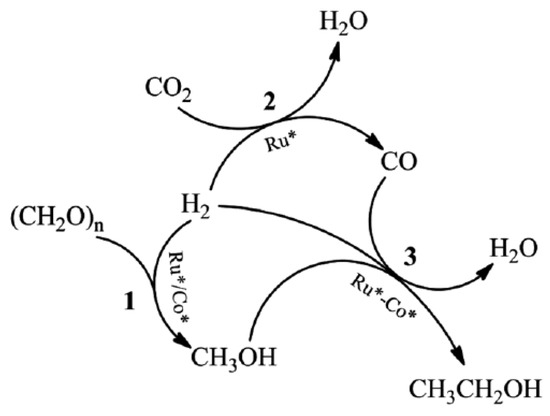
Figure 4. Synergy between the processes used to fabricate ethanol from paraformaldehyde, CO
2, and H
2. The active species of Ru and Co are represented, respectively, by Ru* and Co*. Reprinted with permission from Ref.
[56][150]. Copyright © 2017, Royal Society of Chemistry.
Significant information about the involvement of oxygen in the electrochemical reduction of CO
2 on Cu electrodes was presented in 2017 by Le Duff and colleagues. They also regulated the surface structure and composition of Cu single crystal electrodes over time
[57][151]. Since the pulse sequence may be controlled to ensure consistent beginning conditions for the reaction at every fraction of time and at a certain frequency, this was accomplished using pulsed voltammetry. Compared to the selective CO
2 reduction achieved using cyclic voltammetry
[58][152], and chronoamperometric techniques
[59][153], under alternating voltage, a wide range of oxygenated hydrocarbons was discovered. The coverage of oxygen species, which is reliant on surface structure and potential, was linked to product selectivity towards the synthesis of oxygenated hydrocarbon. A nanowire-like WSe
2-graphene catalyst was created by Ali and Oh in 2017 and examined for its ability to photocatalytically convert CO
2 into CH
3OH when exposed to UV-visible light. XRD, SEM, TEM, Raman, and XPS were used to further characterize the produced nanocomposite. Using gas chromatography (GCMS-QP2010SE), the photocurrent analysis was further evaluated for its photocatalytic reduction of CO
2. The sacrificial agent (Na
2S-Na
2SO
3) was added to WSe
2-graphene nanocomposite to further increase the photocatalytic effectiveness, and it was discovered that this improved the photocatalytic efficiency, with the methanol output reaching 5.0278 mol g
−1 h
−1 [60][154]. In a different study, Biswas et al. (2018) reported using ultrasonic techniques to create a WSe
2-Graphene-TiO
2 ternary nanocomposite
[61][155]. According to estimates, the WSe
2-Graphene-TiO
2 band-gap is 1.62 eV, which is adequate for the photocatalytic degradation when exposed to UV–visible light. For the conversion of CO
2 to CH
3OH, the photocatalytic capability of nanocomposites was examined. After 48 h, WSe
2-G-TiO
2 with an optimal graphene loading of 8 wt% shown high photoactivity, yielding a total CH
3OH yield of 6.3262 mol g
−1 h
−1. The gradual synergistic relationship between the WSe
2-TiO
2 and graphene components in the heterogeneous system is what causes this exceptional photoreduction activity. Ethylene could be produced from CO
2 electroreduction; however, present systems are constrained by low conversion efficiency, slow production rates, and unstable catalysts. In contrast to a reversible hydrogen electrode (RHE), Dinh et al. (2018) found that a copper electrocatalyst at an abrupt reaction interface in an alkaline electrolyte converts CO
2 to ethylene with a 70% faradaic efficiency (FE) at a potential of −0.55 volts
[62][156]. The activation energy barriers for CO
2 reduction and carbon monoxide (CO)-CO coupling are lowered by hydroxyl ions on or near the copper surface, and as a result, ethylene evolution begins at −0.165 volts versus an RHE in 10 molar potassium hydroxide virtually concurrently with CO generation. By sandwiching the reaction interface between different hydrophobic and conductive supports, a polymer-based gas diffusion layer was introduced to increase operational stability while maintaining continuous ethylene selectivity over the first 150 operating hours. In 2019, Ma and Porosoff proposed reaction mechanisms by combining in situ characterization techniques with DFT calculations, identifying structure–property relationships for the zeolite support, strategizing methods to increase catalyst lifetime, and developing advanced synthesis techniques for depositing a metal-based active phase within a zeolite for highly active, selective, and stable tandem catalysts
[63][157]. The critical research topics of reaction mechanism elucidation by combining in situ characterization methods with density functional theory calculations, identifying structure–property relationships for the zeolite support, developing advanced synthesis techniques for depositing a metal-based active phase within a zeolite for highly active, selective, and stable tandem cations, and strategizing methods to extend catalyst lifetime, are suggested as future research directions. An appealing method for storing such a renewable energy source in the form of chemical energy is the conversion of CO
2 into hydrocarbons using solar energy. A system that couples a photovoltaic (PV) cell to an electrochemical cell (EC) for CO
2 reduction can do this. Such a system should have minimum energy losses related to the catalysts at the anode and cathode, as well as the electrolyzer device, in order to be advantageous and usable. It should also use inexpensive and easily processed solar cells. All of these factors were taken into account by Huan et al. in 2019 when setting up a reference PV-EC system for CO
2 reduction to hydrocarbons
[64][158]. Combined with a fairly priced, state-of-the-art perovskite photovoltaic minimodule, this system sets a standard for a low-cost, all-earth-abundant PV-EC system with a solar-to-hydrocarbon efficiency of 2.3%. In 2019, Wu et al. demonstrated cobalt phthalocyanine (CoPc) catalysis for the six-electron reduction of CO
2 to methanol with considerable activity and selectivity when immobilized on CNTs
[65][159]. They discovered that the conversion produces methanol with FE > 40% and a partial current density exceeding 10 mm/cm
2 at −0.94 volts with respect to the reversible hydrogen electrode in a near-neutral electrolyte. CO serves as an intermediary in a special domino mechanism that moves the conversion along. By adding amino substituents that donate electrons to the phthalocyanine ring, it is possible to prevent the harmful reduction of the phthalocyanine ligand from having a negative effect on the catalytic activity. With significant activity, selectivity, and stable performance for at least 12 h, the enhanced molecule-based electrocatalyst converts CO
2 to methanol.
A novel multifunctional catalyst made of Fe
2O
3 encapsulated in K
2CO
3 was introduced by Ramirez et al. in 2019 and has the ability to use a tandem process to convert CO
2 into olefins
[66][160]. The authors established that, unlike the conventional systems in FT processes, very large K loadings are essential to activate CO
2 via the well-known “potassium carbonate mechanism.” While utilizing CO
2 as a feedstock, the suggested catalytic process proved to be just as productive as currently used commercial synthesis gas-based techniques. By employing Cu-doped MgAl
2O
4 (Mg
1−xCu
xAl
2O
4) and a straightforward deposition–reduction process, Tada et al. in 2020 investigated the synthesis of Cu NPs. The following three Cu
2+ species were present in Mg
1−xCu
xAl
2O
4 [67][161]: short O-Cu octahedrally coordinated [CuO
6]
s, elongated O-Cu octahedrally coordinated [CuO
6]
el, and tetrahedrally coordinated [CuO
4]
t. The first two were discovered in Mg
1−xCu
xAl
2O
4 of the inverse-spinel type, and the third was discovered in Mg
1−xCu
xAl
2O
4 of the normal-spinel type. In addition, they made it clear that their percentage is related to Cu loading by concentrating on the variation in the reducibility of the Cu
2+ species. Mg
1−xCu
xAl
2O
4 predominantly comprised the [CuO
6]
s species at low Cu loading (x < 0.3). In contrast, the fraction of the [CuO
6]
el and [CuO
4]
t species rose with high Cu loading (x ≥ 0.3). Notably, the H
2-reduced Mg
0.8Cu
0.2Al
2O
4 (x = 0.2) catalyst showed the best photocatalytic activity among the synthesized catalysts because it had the most exposed metallic Cu sites. Therefore, the formation of metallic Cu NPs on metal oxides depends on the H
2 reduction of [CuO
6]
s.
In 2021, Tada et al. suggested bifunctional tandem ZnZrO
x catalysts for the hydrogenation of CO
2 to methanol along with a number of solid acid catalysts (for subsequent methanol conversion to light olefins)
[68][162]. Researchers used zeolites and silicoaluminophosphates with a variety of topologies, including MOR, FER, MFI, BEA, CHA, and ERI, as solid acid catalysts. A study using ammonia adsorption revealed that they likewise displayed the equivalent acid characteristics. Lower olefins were being synthesized in one step using the tandem catalysts, whereas with ZnZrO
x alone, no hydrocarbons could be produced. There appears to be no relationship between product yields and acid strength, at least according to the reaction test and ammonia adsorption. The product selectivity is influenced by the pore sizes and the channel dimensionality of the zeolites; zeolites with small pores, like MOR, SAPO-34, and ERI, are promising, whereas zeolites with bigger pores, like MFI, generate heavier hydrocarbons. The outcomes offer fresh perspective on the creation of creative catalysts for CO
2 usage. A low-temperature atmospheric surface dielectric barrier discharge reactor that converts biogas into liquid chemicals was introduced by Rahmani et al. in 2021
[69][163]. The effect of steam on the conversion of methane and CO
2 was investigated, as well as the distribution of products in relation to a given energy input based on the operational circumstances. The authors reported conversion rates of 44% for CH
4 and 22% for CO
2. When steam was introduced at the in-feed, the lowest energy cost of 26 eV/molecule was attained. For liquid hydrocarbons, a selectivity of 3% was attained. The transformation of biogas (CH
4 + CO
2) resulted in the production of more than 12 compounds. At ambient temperature, the most prevalent oxygenated hydrocarbon liquids were acetone, methanol, ethanol, and isopropanol. H
2, CO, C
2H
4, and C
2H
6 were the major gases produced.
In order to explain an alternative approach for the chemical CO
2 reduction reaction, Islam et al. in 2021 subjected up to 3% CO
2-saturated pure water, NaCl, and artificial seawater solutions to high-power ultrasound (488 kHz ultrasonic plate transducer)
[70][164]. The converted CO
2 products under ultrasonic settings were discovered to be mostly CH
4, C
2H
4, and C
2H
6, as well as a significant amount of CO that was later converted into CH
4. The analysis revealed that adding molecular H
2 to the CO
2 conversion process is essential, and that raising the hydrogen concentration boosted hydrocarbon yields. However, it was found that the overall conversion decreased at higher hydrogen concentrations because hydrogen, a diatomic gas, is known to reduce cavitation activity in liquids. Additionally, it was discovered that the maximum hydrocarbon yields (nearly 5%) were achieved with 1.0 M NaCl solutions saturated with 2% CO
2 + 98% H
2, and that increasing salt concentrations further decreased the yield of hydrocarbons due to the combined physical and chemical effects of ultrasound. By diluting the flue gas with hydrogen, it was demonstrated that the CO
2 present in a synthetic industrial flue gas (86.74% N
2, 13.5% CO
2, 0.2% O
2, and 600 ppm of CO) could be transformed into hydrocarbons. Additionally, it has been demonstrated that the conversion process can be carried out in ambient circumstances, i.e., at room temperature and pressure, without the use of catalysts, when low-frequency, high-power ultrasound is used. Tian et al. in 2022 newly manufactured In
2O
3, MnO-In
2O
3, and MgO-In
2O
3 catalysts using the co-precipitation method, and they looked into the hydrogenation of CO
2 to methanol
[71][165]. The ability of In
2O
3 to absorb CO
2 was significantly improved by the addition of Mn and Mg oxides. The CO
2 adsorption capacity and the changing trend of methanol selectivity were consistent. As opposed to In
2O
3, the methanol selectivity of MnO-In
2O
3 and MgO-In
2O
3 catalysts is higher. ODP, or oxidative dehydrogenation of propane with CO
2, is a promising solution for efficient CO
2 usage. In a new study published in 2022, Chernyak et al. included various C-materials for the first time as supports for Cr-based catalysts of CO
2-assisted ODP
[72][166]. A commercially available activated carbon was evaluated alongside CNTs, jellyfish-like graphene nanoflakes, and their oxidized and N-doped derivatives. The oxidized CNT- and pure GNF-supported catalysts showed the highest activity and a propylene yield of up to 25%. Raman spectroscopy was used to confirm that these two catalysts were stable throughout tests against disintegration and particle sintering. The oxidized CNT- and pristine graphene nanoflakes-supported catalysts’ high activity and durability were explained by their macro- and mesoporosity, which improve reagent and product diffusion, as well as by the highest surface graphitization degree, which was validated by XPS. These catalysts performed significantly better than the catalyst supported by activated carbon. As a result, CNTs and graphene nanoflakes are suitable supports for CO
2-ODP catalytic compounds. Several main catalysts used in the hydrogenation of CO
2 to hydrocarbon were summarized in
Table 1.
Table 1. Some of the main catalysts used in the hydrogenation of CO2 to hydrocarbon products.
Although the technique of hydrogenating carbon dioxide to produce methanol is inexpensive and environmentally benign, nevertheless, because of the increased stability and inertness of CO2, this reaction is thermodynamically constrained. The reaction below reveals the process of methanol production.
The reaction is thermodynamically advantageous at low temperatures and high pressure, according to the Le Chatelier’s principle. The enhanced thermal stability and chemical inertness of CO
2 means that this method of methanol synthesis requires a high temperature to proceed. Indeed, at elevated reaction temperatures (for example, higher than 240 °C), CO
2 activation and subsequent methanol production are facilitated. However, the higher temperature procedure strongly conflicts with the reaction’s thermodynamics. Furthermore, when the reaction is conducted at a higher temperature, undesired byproducts including higher alcohols and hydrocarbons are formed. Similar to this, because the CO
2 hydrogenation process produces methanol, which is a molecular reducing reaction, it is thermodynamically more advantageous at high pressure. By employing various catalysts, various reaction pressure sizes have been suggested for the best CO
2 conversion
[46][140].