A probiotic is a live microorganism that improves the host's health when administrated in adequate amounts.
- Probiotics
- acid stress
- atomisation
1. Introduction
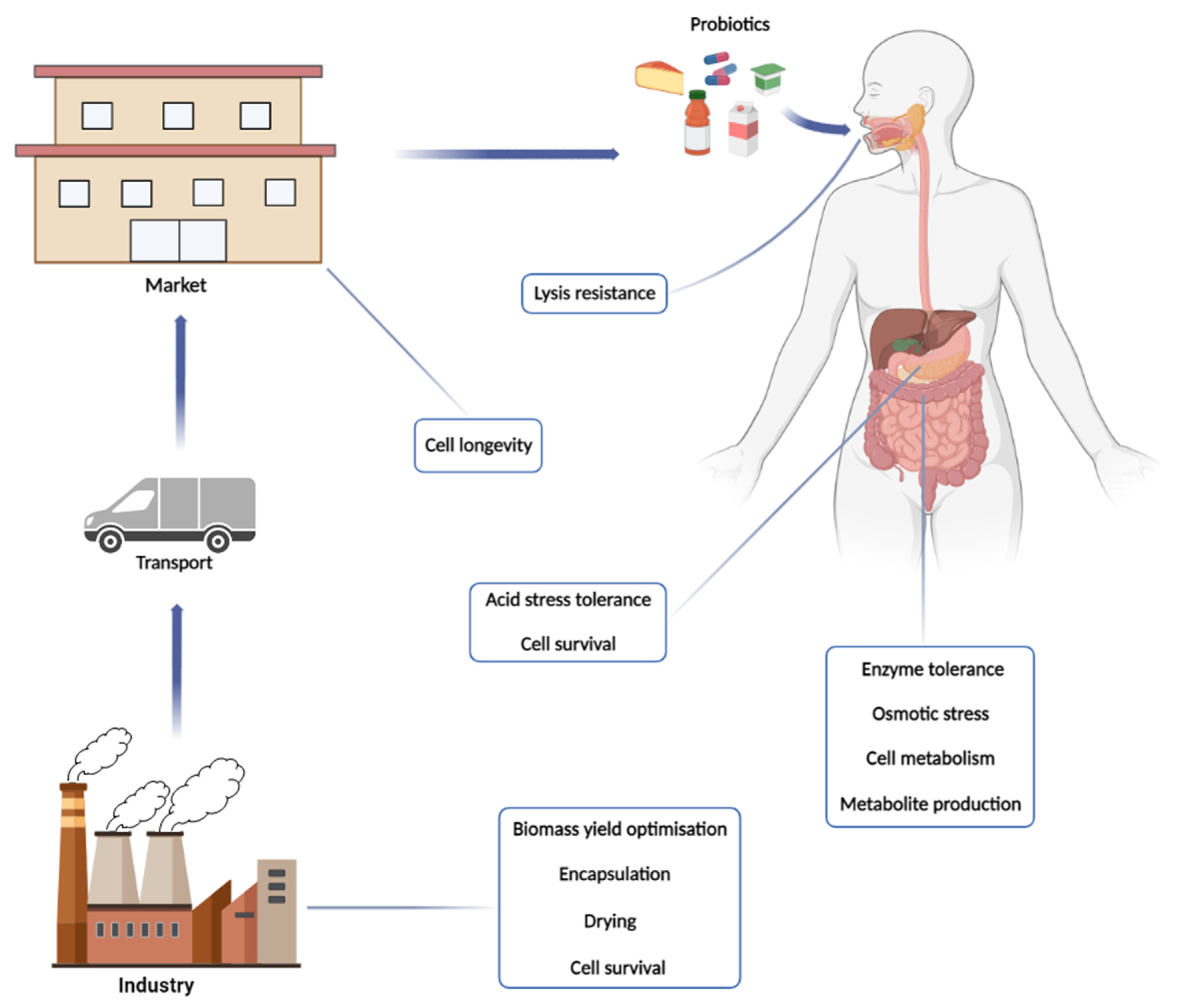
2. The Origin: Evolution of Probiotic Meaning
|
Name |
Definition |
References |
||
|---|---|---|---|---|
|
Substances produced by microorganisms that promote the growth of other microorganisms. |
[7 | |||
|
Paraprobiotic | ] |
|||
“Dead bacterial cells or cell components to denote health benefits beyond the inherent viability of probiotics.” |
[20] |
“Organisms and substances which contribute to intestinal microbial balance.” |
[8] |
|
|
Postbiotic |
Any substance released by or produced through the metabolic activity of the microorganism, which exerts a beneficial effect on the host, directly or indirectly. |
[21] |
“A live microbial feed supplement that beneficially affects the host animal by improving its intestinal microbial balance.” |
[ |
|
Prebiotics | 9] |
|||
“Substrate that is selectively utilized by host microorganisms conferring a health benefit.” |
[16 |
“Living micro-organisms administered in a sufficient number to survive in the intestinal ecosystem. They must have a positive effect on the host.” |
[10] |
|
] |
“A mono or mixed culture of living microorganisms that benefit humans or animals by improving the properties of the indigenous microflora.” |
[11] |
||
|
“Living micro-organisms, which upon ingestion in certain numbers, exert health benefits beyond inherent basic nutrition.” |
||||
|
Synbiotic |
Mixture of prebiotics and probiotics for the improvement of human or animal health. |
[22] |
[12] |
|
|
“Live microorganisms which when administered in adequate amounts confer a health benefit on the host.” |
[13] |
3. Screening and Selection
4. Applications in Contemporary Times
References
- Ozen, M.; Dinleyici, E.C. The History of Probiotics: The Untold Story. Benef. Microbes. 2015, 6, 159–165.
- Godoy, A.; Herrera, T.; Ulloa, M. Más Allá Del Pulque y El Tepache: Las Bebidas Alcohólicas No Destiladas Indígenas de México; 1a; Universidad Nacional Autonoma de México: Ciudad de México, Mexico, 2003.
- Nakazawa, Y.; Hosono, A. (Eds.) Functions of Fermented Milk: Challenges for the Health Sciences. In Fermented Milk in the Orient; Elsevier Applied Science: London, UK, 1992; pp. 61–78.
- Abou-Donia, S.A. Origin, History and Manufacturing Process of Egyptian Dairy Products: An Overview. Alex. J. Food Sci. Technol. 2008, 5, 51–62.
- Tissier, H. Recherches Sur La Flore Intestinale Des Nourrissons: (État Normal et Pathologique); Méd.—Paris: Paris, France, 1900.
- Shokryazdan, P.; Faseleh Jahromi, M.; Liang, J.B.; Ho, Y.W. Probiotics: From Isolation to Application. J. Am. Coll. Nutr. 2017, 36, 666–676.
- Lilly, D.M.; Stillwell, R.H. Probiotics: Growth-Promoting Factors Produced by Microorganisms. Science 1965, 147, 747–748.
- Parker, R.B. Probiotics, the Other Half of the Antibiotic Story. Anim. Nutr. Health 1974, 29, 4–8.
- Fuller, R. Probiotics in Man and Animals. J. Appl. Bacteriol. 1989, 66, 365–378.
- Gismondo, M.R.; Drago, L.; Lombardi, A. Review of Probiotics Available to Modify Gastrointestinal Flora. Int. J. Antimicrob. Agents 1999, 12, 287–292.
- Havenaar, R.; Huis In’t Veld, J.H.J. Probiotics: A General View. In The Lactic Acid Bacteria Volume 1; Springer US: Boston, MA, USA, 1992; pp. 151–170.
- Guarner, F. Probiotics. Int. J. Food Microbiol. 1998, 39, 237–238.
- FAO; WHO. Guidelines for the Evaluation of Probiotics in Food; FAO: London, ON, Canada, 2002.
- NIH Probiotics: What You Need to Know. Available online: https://www.nccih.nih.gov/health/probiotics-what-you-need-to-know (accessed on 8 November 2022).
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; da Cruz, A.G. Probiotic: Conceptualization from a New Approach. Curr. Opin. Food Sci. 2020, 32, 103–123.
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502.
- Silva, D.R.; Sardi, J.D.C.O.; Pitangui, N.D.S.; Roque, S.M.; da Silva, A.C.B.; Rosalen, P.L. Probiotics as an Alternative Antimicrobial Therapy: Current Reality and Future Directions. J. Funct. Foods 2020, 73, 104080.
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, Prebiotics and Synbiotics: Safe Options for next-Generation Therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521.
- Ma, J.; Lyu, Y.; Liu, X.; Jia, X.; Cui, F.; Wu, X.; Deng, S.; Yue, C. Engineered Probiotics. Microb. Cell Fact. 2022, 21, 72.
- Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and Paraprobiotics: From Concepts to Applications. Food Res. Int. 2020, 136, 109502.
- Tsilingiri, K.; Rescigno, M. Postbiotics: What Else? Benef. Microbes 2013, 4, 101–107.
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021.
- Baker, D.D.; Chu, M.; Oza, U.; Rajgarhia, V. The Value of Natural Products to Future Pharmaceutical Discovery. Nat. Prod. Rep. 2007, 24, 1225.
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural Products for Human Health: An Historical Overview of the Drug Discovery Approaches. Nat. Prod. Res. 2018, 32, 1926–1950.
- Arnold, C. The New Danger of Synthetic Drugs. Lancet 2013, 382, 15–16.
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog Glob. Health 2015, 109, 309–318.
- Trasande, L.; Shaffer, R.M.; Sathyanarayana, S.; Lowry, J.A.; Ahdoot, S.; Baum, C.R.; Bernstein, A.S.; Bole, A.; Campbell, C.C.; Landrigan, P.J.; et al. Food Additives and Child Health. Pediatrics 2018, 142, e20181410.
- Nguyen, M.; Holdbrooks, H.; Mishra, P.; Abrantes, M.A.; Eskew, S.; Garma, M.; Oca, C.-G.; McGuckin, C.; Hein, C.B.; Mitchell, R.D.; et al. Impact of Probiotic B. Infantis EVC001 Feeding in Premature Infants on the Gut Microbiome, Nosocomially Acquired Antibiotic Resistance, and Enteric Inflammation. Front. Pediatr. 2021, 9, 618009.
- Sowden, M.; van Niekerk, E.; Bulabula, A.N.H.; Dramowski, A.; Whitelaw, A.; Twisk, J.; van Weissenbruch, M.M. Impact of a Multi-Strain Probiotic Administration on Peri-Rectal Colonization with Drug-Resistant Gram-Negative Bacteria in Preterm Neonates. Front. Pediatr. 2022, 10, 1002762.
- Damaceno, Q.S.; Gallotti, B.; Reis, I.M.M.; Totte, Y.C.P.; Assis, G.B.; Figueiredo, H.C.; Silva, T.F.; Azevedo, V.; Nicoli, J.R.; Martins, F.S. Isolation and Identification of Potential Probiotic Bacteria from Human Milk. Probiotics Antimicrob. Proteins 2021.
- Santos, D.D.S.; Calaça, P.R.D.A.; Porto, A.L.F.; de Souza, P.R.E.; de Freitas, N.S.A.; Soares, M.T.C.V. What Differentiates Probiotic from Pathogenic Bacteria? The Genetic Mobility of Enterococcus Faecium Offers New Molecular Insights. Omics 2020, 24, 706–713.
- Bank, N.C.; Singh, V.; Rodriguez-Palacios, A. Classification of Parabacteroides Distasonis and Other Bacteroidetes Using O- Antigen Virulence Gene: RfbA -Typing and Hypothesis for Pathogenic vs. Probiotic Strain Differentiation. Gut Microbes 2022, 14, 1997293.
- Asadi, A.; Lohrasbi, V.; Abdi, M.; Mirkalantari, S.; Esghaei, M.; Kashanian, M.; Oshaghi, M.; Talebi, M. The Probiotic Properties and Potential of Vaginal Lactobacillus Spp. Isolated from Healthy Women against Some Vaginal Pathogens. Lett. Appl. Microbiol. 2022, 74, 752–764.
- Ouwehand, A.C.; Kirjavainen, P.v.; Shortt, C.; Salminen, S. Probiotics: Mechanisms and Established Effects. Int. Dairy J. 1999, 9, 43–52.
- Lin, M.Y.; Chang, F.J. Antioxidative Effect of Intestinal Bacteria Bifidobacterium Longum ATCC 15708 and Lactobacillus Acidophilus ATCC 4356. Dig. Dis. Sci. 2000, 45, 1617–1622.
- Pereira, D.I.A.; Gibson, G.R. Cholesterol Assimilation by Lactic Acid Bacteria and Bifidobacteria Isolated from the Human Gut. Appl. Environ. Microbiol. 2002, 68, 4689–4693.
- Thirabunyanon, M.; Boonprasom, P.; Niamsup, P. Probiotic Potential of Lactic Acid Bacteria Isolated from Fermented Dairy Milks on Antiproliferation of Colon Cancer Cells. Biotechnol. Lett. 2009, 31, 571–576.
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The Pros, Cons, and Many Unknowns of Probiotics. Nat. Med. 2019, 25, 716–729.
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858.
- Probiotics Market Size, Share & Trends Analysis Report By Product (Probiotic Food & Beverages, Probiotic Dietary Supplements), By Ingredient (Bacteria, Yeast), By End Use, By Distribution Channel, And Segment Forecasts, 2021–2030. Available online: https://www.grandviewresearch.com/industry-analysis/probiotics-market/toc (accessed on 8 November 2022).
- Westermann, C.; Gleinser, M.; Corr, S.C.; Riedel, C.U. A Critical Evaluation of Bifidobacterial Adhesion to the Host Tissue. Front Microbiol. 2016, 7, 1220.
- Ranadheera, C.; Vidanarachchi, J.; Rocha, R.; Cruz, A.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67.
- Souza, H.F.; Carosia, M.F.; Pinheiro, C.; de Carvalho, M.V.; de Oliveira, C.A.F.; Kamimura, E.S. On Probiotic Yeasts in Food Development: Saccharomyces Boularrdii, a Trend. Food Sci. Technol. 2022, 42.
- George Kerry, R.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of Probiotics for Human Health: A Review. J. Food Drug Anal. 2018, 26, 927–939.
- de Araújo Henriques Ferreira, G.; Magnani, M.; Cabral, L.; Brandão, L.R.; Noronha, M.F.; de Campos Cruz, J.; de Souza, E.L.; de Brito Alves, J.L. Potentially Probiotic Limosilactobacillus Fermentum Fruit-Derived Strains Alleviate Cardiometabolic Disorders and Gut Microbiota Impairment in Male Rats Fed a High-Fat Diet. Probiotics Antimicrob. Proteins 2022, 14, 349–359.
- Moreira, C.F.; Cassini-Vieira, P.; Canesso, M.C.C.; Felipetto, M.; Ranfley, H.; Teixeira, M.M.; Nicoli, J.R.; Martins, F.S.; Barcelos, L.S. Lactobacillus Rhamnosus CGMCC 1.3724 (LPR) Improves Skin Wound Healing and Reduces Scar Formation in Mice. Probiotics Antimicrob. Proteins 2021, 13, 709–719.
- Li, P.; Ji, B.; Luo, H.; Sundh, D.; Lorentzon, M.; Nielsen, J. One-Year Supplementation with Lactobacillus Reuteri ATCC PTA 6475 Counteracts a Degradation of Gut Microbiota in Older Women with Low Bone Mineral Density. NPJ Biofilms Microbiomes 2022, 8, 84.
- Da Silva, T.F.; Casarotti, S.N.; De Oliveira, G.L.V.; Penna, A.L.V. The impact of probiotics, prebiotics, and synbiotics on the biochemical, clinical, and immunological markers, as well as on the gut microbiota of obese hosts. Crit. Rev. Food Sci. Nutr. 2021, 61, 337–355.
- Dinkçi, N.; Akdeniz, V.; Akalin, A.S. Survival of Probiotics in Functional Foods during Shelf Life. In Food Quality and Shelf Life; Elsevier: Amsterdam, The Netherlands, 2019; pp. 201–233.
- Mushtaq, M.; Gani, A.; Masoodi, F.A. Himalayan Cheese (Kalari/Kradi) Fermented with Different Probiotic Strains: In Vitro Investigation of Nutraceutical Properties. LWT 2019, 104, 53–60.
- Rolim, F.R.L.; Freitas Neto, O.C.; Oliveira, M.E.G.; Oliveira, C.J.B.; Queiroga, R.C.R.E. Cheeses as Food Matrixes for Probiotics: In Vitro and in Vivo Tests. Trends Food Sci. Technol. 2020, 100, 138–154.
- Nematollahi, A.; Sohrabvandi, S.; Mortazavian, A.M.; Jazaeri, S. Viability of Probiotic Bacteria and Some Chemical and Sensory Characteristics in Cornelian Cherry Juice during Cold Storage. Electron. J. Biotechnol. 2016, 21, 49–53.
- dos Santos Nascimento, D.; Sampaio, K.B.; do Nascimento, Y.M.; de Souza, T.A.; de Souza, F.S.; Júnior, J.V.C.; Tavares, J.F.; da Silva, M.S.; de Brito Alves, J.L.; de Souza, E.L. Evaluating the Stability of a Novel Nutraceutical Formulation Combining Probiotic Limosilactobacillus Fermentum 296, Quercetin, and Resveratrol Under Different Storage Conditions. Probiotics Antimicrob. Proteins 2022.
- Kim, C.E.; Yoon, L.S.; Michels, K.B.; Tranfield, W.; Jacobs, J.P.; May, F.P. The Impact of Prebiotic, Probiotic, and Synbiotic Supplements and Yogurt Consumption on the Risk of Colorectal Neoplasia among Adults: A Systematic Review. Nutrients 2022, 14, 4937.
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Role of Probiotics and Diet in the Management of Neurological Diseases and Mood States: A Review. Microorganisms 2022, 10, 2268.
- Wilmink, J.M.; Ladefoged, S.; Jongbloets, A.; Vernooij, J.C.M. The Evaluation of the Effect of Probiotics on the Healing of Equine Distal Limb Wounds. PLoS ONE 2020, 15, e0236761.
- Albrecht, E.; Zitnan, R.; Karaffova, V.; Revajova, V.; Čechová, M.; Levkut, M., Jr.; Röntgen, M. Effects of the Probiotic Enterococcus Faecium on Muscle Characteristics of Chickens. Life 2022, 12, 1695.
- Várhidi, Z.; Máté, M.; Ózsvári, L. The Use of Probiotics in Nutrition and Herd Health Management in Large Hungarian Dairy Cattle Farms. Front. Vet. Sci. 2022, 9, 957935.
- de Oliveira, K.Á.R.; Fernandes, K.F.D.; de Souza, E.L. Current Advances on the Development and Application of Probiotic-Loaded Edible Films and Coatings for the Bioprotection of Fresh and Minimally Processed Fruit and Vegetables. Foods 2021, 10, 2207.
