Botanically, cocoa (Theobroma cacao) is a typical evergreen tree belonging to the Sterculiaceae family, originally from tropics of Central and South America and then grown in the other parts of the world. The plant has three main cultivars, with different phytochemical content and sensory properties: Criollo, the first cultivated group, Forastero, the hardier and the most prevalent group, and Trinitario, a cross-breed of both Criollo and Forastero groups.
- cocoa
- polyphenols
- flavanols
- bioavailability
- gut microbiota
- human health
1. Introduction
Cocoa and its derivatives are abundantly consumed worldwide due to their pleasant taste and numerous functional effects. To date, several studies have demonstrated that consumption of cocoa and its products decreases the risk of cardiovascular diseases and metabolic disorders, positively affects the immune and nervous systems, prevents the risk of cancer, and shows systemic and intestinal anti-inflammatory activities
[1]
. Cocoa phytochemical constituents, particularly polyphenols, are strongly associated with health-promoting benefits. Cocoa polyphenols have effective antioxidant and anti-inflammatory properties, interacting with various relevant pathways associated with several health advantages. For instance, flavanol-rich cocoa and cocoa derivatives increase nitric oxide (NO) synthesis, augment flow-mediated dilation (FMD) and enhance microcirculation
, induce vasodilation and reduce blood pressure
[4]
, attenuate platelet aggregation and improve endothelial and vascular function
.
2. Cocoa Composition and Processing
Every year, millions of tons of cocoa are produced. The seeds of the fruits, cocoa beans, are then processed into cocoa powder, butter, and liquor
[9]
. Cocoa beans contain water, lipids, proteins, fibers, and many biologically active compounds
[10]
. In total, around 380 compounds have been identified in cocoa
[1]
. Among them, polyphenols and xanthine alkaloids are predominant, comprising nearly 14–20% of the bean weight
[11]
. Cocoa processing leads to a vast decrease in phytochemical concentration and changes their proportion
[1]
. In plants, phenolic compounds are involved in antioxidant activity, protection from environmental stressors such as UV radiation, microbial and fungal infection, and accordingly aiding the plant development
[12]
.
Cocoa is especially abundant in flavanols, accounting for around 60% in non-fermented cocoa beans
[13]
. Cocoa flavanol comprises monomeric forms, (+)-catechin and (−)-epicatechin, and their oligomeric and polymeric forms, procyanidins. The types cocoa flavanols entail (−)–epicatechin, being the most abundant, (+)−catechin, procyanidin B1, and B2, and other flavanols at trace level, such as epigallocatechin, epigallocatechin-3-gallate, procyanidin B2-O-gallate, procyanidin B2-3,3-di-O-gallate, procyanidin B3, procyanidin B4, procyanidin B4-O-gallate, procyanidin C1, and procyanidin D (Table 1)
[14]
. Other minor polyphenolic compounds include flavones (luteolin, luteolin-7-O-glucoside, orientin, isoorientin, apigenin, vitexin, and isovitexin), flavanones (naringenin, prunin, hesperidin, and eriodictyol), flavonols (quercetin, quercetin-3-O-arabinoside, isoquercitrin, and hyperoside), anthocyanidins (cyanidin, 3-α-l-arabinosidyl cyanidin, 3-β-d-arabinosidyl cyanidin, 3-β-D-galactosidyl cyanidin), and certain phenolic acids (Figure 1)
[15]
.
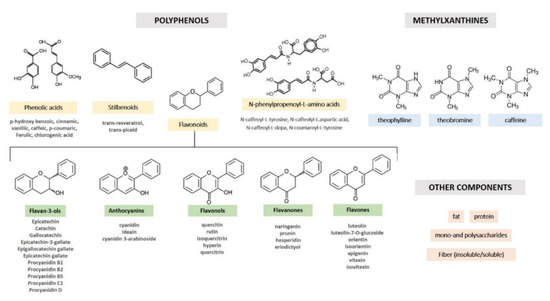
Figure 1.
Nomenclature and chemical structures of cocoa phytocomplex.
Table 1.
Phytochemicals’ concentrations in cocoa powder.
| Phytochemical | Mg/g of Fresh Weight | Reference |
|---|
| Flavanols | (−)-Epicatechin | 0.63- 3.3 | [16][17] |
| (+)-Catechin | 0.61–2.02 | [18][17] | |
| Procyanidin B1 (dimer) | 1.12 | [17] | |
| Procyanidin B2 (dimer) | 0.13- 2.62 | [16][17] | |
| Procyanidin C1 (trimer) | 0.05- 0.36 | [16] | |
| Cinnamtannin A2 (tetramer) | 0.31–0.56 | [16] | |
| Galactopyranosyl-ent-(−)-epicatechin (2alpha-->7, 4alpha->8)-(−)-epicatechin (Gal-EC-EC) | 0.02–0.07 | [16] | |
| Flavonols | Quercetin | 0.0032–0.2 | [19][20] |
| Isoquercitrin | 0.03334–0.04664 | [19] | |
| Quercetin-3-Arabinoside | 0.05258–0.09212 | [19] | |
| Quercetin 3-O-glucuronide | 0.00938–0.01512 | [19] | |
| Phenolic acids | |||
| Hydroxybenzoic acids | Benzoic acid | 0.00017–0.00097 | [21] |
| Hydroxycinnamic acids | Caffeoyl aspartate | 0.37 | [17] |
| Hydroxybenzaldehydes | Vanillin | 0.00014–0.00232 | [21] |
| Methylxanthines | Theobromine | 4.11–27.69 | [22][23] |
| Caffeine | 0.946–6.58 | [22][23] | |
| Others | Catechol | 0.0012 | [24] |
| Pyrogallol | 0.0018 | [24] |
Cocoa polyphenolic profile and concentration varies depending on the type of cultivar, quality of crop and cultivation site, geographic area, and the climate. Its content also considerably varies among different cocoa products, influenced by processing and manufacturing steps
. To estimate their variations, researchers examined the polyphenol profiles and antioxidant properties of different cocoa liquor samples from six different geographic places (Madagascar, Mexico, Ecuador, Venezuela, Sao Tome, and Ghana). They observed that similar types of polyphenol compounds are present, but their proportion and antioxidant activities are different. Among them, (−)-epicatechin ranged from 0.16 to 0.59 mg/g defatted cocoa liquorsDCL and (+)-catechin varied between 0.02 and 0.42 mg/g DCL. (−)-Gallocatechin was not found in Ghana and Venezuela cocoa liquors but in other samples ranged from 0.06 to 0.40 mg/g DCL. Likewise, (−)-epigallocatechin content estimated to vary between 0.15 and 0.42 mg/g DCL, although it was not detected in Mexico and Venezuela samples. Overall, total polyphenolic contents were in the following order: Madagascar > Mexico > Ecuador > Venezuela > Sao Tome > Ghana. They also observed that higher polyphenolic content corresponds to higher antioxidant activity, indicating the contributing role of polyphenols in the antioxidant property of cocoa
[11]
.
The production of cocoa products from cocoa beans takes several steps, and its chemistry changes by each step of the processing. It starts with drying and a five-to-seven day of fermentation, which is carried out in specific containers with a temperature of 45–50 Celsius
[9]
. The beans are then broken to separate the nib from its shell and subsequently sterilized. This is followed by alkalization process by an alkali solution of potassium or sodium carbonate at a temperature of 80–100 Celsius
[25]
. Subsequently, the alkalized product is roasted and ground to reduce the nibs to liquor or pressed to separate the fat content from the powder and eventually to produce cocoa powder and butter
[26]
. These final products are widely used to make chocolate, candies, and other cocoa derivatives.
Fermentation is an essential step in cocoa bean processing, during which cocoa polyphenols are oxidized enzymatically by polyphenol oxidase or non-enzymatically; then, they are polymerized and bind with proteins, resulting in high-molecular compounds (tannins) with reduced solubility and astringency. Eventually, these alterations contribute to the final product color and flavor
. During this step, epicatechins and soluble polyphenols reduce by 10–20% due to oxidation and cocoa sweating. Anthocyanins, which gradually vanish during fermentation, are used as an index for determining the fermentation degree since they hydrolyze into anthocyanidins and polymerize with catechins to form tannins. Likewise, procyanidin level and fermentation degree are negatively associated, with their levels being reported to decrease three to five-fold once cocoa is fermented
[29]
.
On the other hand, during fermentation, the total polyphenol amount is positively influenced by the presence of lactic acid bacteria, acetic acid bacteria, and yeasts. In contrast, molds and aerobic spores have the opposite effect. Notably, it is described that a more extended fermentation period leads to the growth of fungi and the production of aerobic spores, which in turn have a negative impact on polyphenol quantity and quality
[27]
.
Considerable loss of cocoa polyphenols takes place during drying, alkalization, and roasting as well
[11]
. During drying, the polyphenol content is substantially reduced by enzymatic browning. For instance, a 2-day drying of cocoa beans causes a 50% reduction in epicatechin [34]. Depending on temperature and timing, the roasting step also results in reduced astringent taste due to the degradation of the polyphenols [31]. In a study, researchers observed that (−)-epicatechin and (+)-catechin enormously decreased once cocoa fermented, while the enantiomer, (−)-catechin, is formed. In the same manner, high-level roasting reduced (−)-epicatechin and (+)-catechin and increased (−)-catechin and alkalization led to a continuous decrease of the monomeric flavanols and a lesser loss of (−)-catechin
[30]
.
In a study to determine the changes during cocoa processing, researchers noticed that roasting and alkalization of cocoa nibs had a major effect in altering the polyphenol content. Roasting resulted in a 27% loss of (−)-epicatechin, from 2.23 mg/g to 1.63 mg/g, while the (+)-catechin content increased from 0.28 mg/g to 0.33 mg/g, which was suggested to be due to its epimerization into (−)-catechin. Procyanidin B1 decreased by 57%, from 0.28 mg/g to 0.12 mg/g and procyanidin B2 declined by 30%, from 0.63 mg/g to 0.44 mg/g. Grounding of the roasted cocoa beans into cocoa liquor led to decrease in (−)-epicatechin by 25%. Subsequently, preparing cocoa baking chocolate further declined the (−)-epicatechin, (+)-catechin, procyanidin B1 and procyanidin B2 into 0.52 mg/g, 0.24 mg/g, 0.04 mg/g and 0.16 mg/g, respectively. Furthermore, alkalizing and drying the roasted and grounded cocoa beans reduced (−)-epicatechin procyanidin B1 by 64% and 60% to 0.49 mg/g and 0.05 mg/g, respectively. Procyanidin B2 decreased by 80% while catechin increased by 27%
[18]
.
Therefore, depending on the type of cocoa processing, the polyphenol content varies considerably, and this must be considered for cocoa health outcomes (Table 2). Furthermore, information on these profile changes helps to find the optimal conditions to reduce the loss of the polyphenol content during processing.
Table 2.
(+)-catechin and (−)-epicatechin content of cocoa bean and its derivatives.
| Source | Quantity (mg/g) | Reference | |
|---|---|---|---|
| (+)-Catechin | (−)-Epicatechin | ||
| Cocoa bean | |||
| 0.28 | |||
| 2.23 | |||
| [ | 28 | ] | |
| Roasted cocoa bean | |||
| 0.33 | |||
| 1.63 | |||
| [ | 28 | ] | |
| Alkalised cocoa nibs | |||
| 0.26 | |||
| 0.58 | |||
| [ | 28 | ] | |
| Alkalised cocoa powder | |||
| 0.60 | |||
| 0.88 | |||
| [ | 28 | ] | |
| Dried alkalised cocoa nibs | |||
| 0.33 | |||
| 0.49 | |||
| [ | 28 | ] | |
| Cocoa liquor | |||
| 0.34 | |||
| 1.22 | |||
| [ | 28 | ] | |
| Cocoa liquor (Venezuela) | |||
| 0.14 | |||
| 0.74 | |||
| [ | 31 | ] | |
| Cocoa liquor (Brazil) | |||
| 0.63 | |||
| 5.77 | |||
| [ | 31 | ] | |
| Baking chocolate | |||
| 0.24 | |||
| 0.52 | |||
| [ | 28 | ] | |
| Dark chocolate (Albert Heijn) | |||
| 0.1324 | |||
| 0.3274 | |||
| [ | 32 | ] | |
| Dark chocolate (Verkade) | |||
| 0.1075 | |||
| 0.5025 | |||
| [ | 32 | ] | |
| Milk chocolate | |||
| 0.05–0.12 | |||
| 0.18–0.24 | |||
| [ | 18 | ] | |
| Milk chocolate (Albert Heijn) | |||
| 0.0383 | |||
| 0.1249 | |||
| [ | 32 | ] | |
| Milk chocolate (Verkade) | |||
| 0.0269 | |||
| 0.1261 | |||
| [ | 32 | ] | |
| Baking chips | |||
| 0.26–0.50 | |||
| 0.66–1.07 | |||
| [ | 18 | ] | |
| Chocolate candy bar | 0.0217 | 0.0625 | [32] |
References
- Andújar, I.; Recio, M.C.; Giner, R.M.; Ríos, J.L. Cocoa Polyphenols and Their Potential Benefits for Human Health. Oxidative Med. Cell. Longev. 2012, 2012, 906252.
- Fraga, C.G.; Litterio, M.C.; Prince, P.D.; Calabró, V.; Piotrkowski, B.; Galleano, M. Cocoa flavanols: Effects on Vascular Nitric Oxide and Blood Pressure. J. Clin. Biochem. Nutr. 2011, 48, 63–67.
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA 2006, 103, 1024–1029.
- Taubert, D.; Roesen, R.; Lehmann, C.; Jung, N.; Schömig, E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: A randomized controlled trial. JAMA 2007, 298, 49–60.
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and dark chocolate polyphenols: From biology to clinical Applications. Front. Immunol. 2017, 8, 677.
- Scapagnini, G.; Davinelli, S.; Di Renzo, L.; De Lorenzo, A.; Olarte, H.H.; Micali, G.; Cicero, A.F.; Gonzalez, S. Cocoa bioactive compounds: Significance and potential for the maintenance of skin health. Nutrients 2014, 6, 3202–3213.
- Davinelli, S.; Corbi, G.; Righetti, S.; Sears, B.; Olarte, H.H.; Grassi, D.; Scapagnini, G. Cardioprotection by cocoa polyphenols and ω-3 fatty acids: A disease-prevention perspective on aging-associated cardiovascular risk. J. Med. Food 2018, 21, 1060–1069.
- Calzavara-Pinton, P.; Calzavara-Pinton, I.; Arisi, M.; Rossi, M.T.; Scapagnini, G.; Davinelli, S.; Venturini, M. Cutaneous Photoprotective Activity of a Short-term Ingestion of High-Flavanol Cocoa: A Nutritional Intervention Study. Photochem. Photobiol. 2019, 95, 1029–1034.
- Rosane F. Schwan; Alan E. Wheals; The Microbiology of Cocoa Fermentation and its Role in Chocolate Quality. Critical Reviews in Food Science and Nutrition 2004, 44, 205-221, 10.1080/10408690490464104.
- Colombo, M.L.; Pinorini-Godly, M.T.; Conti, A. Botany and Pharmacognosy of the Cacao Tree. In Chocolate and Health; Springer Milan: Milano, Italy, 2012; pp. 41–62
- Ivana Radojčić Redovniković; K. DeLonga; S. Mazor; V. Dragović-Uzelac; M. Carić; J. Vorkapić-Furač; Polyphenolic content and composition and antioxidative activity of different cocoa liquors. Czech Journal of Food Sciences 2009, 27, 330-337, 10.17221/119/2008-cjfs.
- Dugald C. Close; Clare McArthur; Rethinking the role of many plant phenolics - protection from photodamage not herbivores?. Oikos 2002, 99, 166-172, 10.1034/j.1600-0706.2002.990117.x.
- Flores, M.E.J. Cocoa Flavanols: Natural Agents with Attenuating Effects on Metabolic Syndrome Risk Factors. Nutrients 2019, 11, 751.
- Borchers, A.T.; Keen, C.L.; Hannum, S.M.; Gershwin, M.E. Cocoa and chocolate: Composition, bioavailability, and health implications. J. Med. Food 2000, 3, 77–105.
- Martín, M.A.; Ramos, S. Cocoa polyphenols in oxidative stress: Potential health implications. J. Funct. Foods 2016, 27, 570–588.
- Natsume, M.; Osakabe, N.; Yamagishi, M.; Takizawa, T.; Nakamura, T.; Miyatake, H.; Hatano, T.; Yoshida, T. Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal-phase and reversed-phase HPLC. Biosci. Biotechnol. Biochem. 2000, 64, 2581–2587.
- Tomas-Barberan, F.A.; Cienfuegos-jovellanos, E.; Marìn, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerda, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; et al. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J. Agric. Food Chem. 2007, 55, 3926–3935.
- Kenneth B. Miller; David A. Stuart; Nancy L. Smith; Chang Y. Lee; Nancy L. McHale; Judith A. Flanagan; Boxin Ou; W Jeffrey Hurst; Antioxidant Activity and Polyphenol and Procyanidin Contents of Selected Commercially Available Cocoa-Containing and Chocolate Products in the United States. Journal of Agricultural and Food Chemistry 2006, 54, 4062-4068, 10.1021/jf060290o.
- Andres-Lacueva, C.; Monagas, M.; Khan, N.; Izquierdo-Pulido, M.; Urpi-Sarda, M.; Permanyer, J.; Lamuela-Raventós, R.M. Flavanol and flavonol contents of cocoa powder products: Influence of the manufacturing process. J. Agric. Food Chem. 2008, 56, 3111–3117.
- Larson, A.; Symons, J.; Jalili, T. Therapeutic Potential of Quercetin to Decrease Blood Pressure: Review of Efficacy and Mechanisms. Adv. Nutr. (Bethesda, Md.) 2012, 3, 39–46.
- Ulrich Krings; Kateryna Zelena; Shimin Wu; Ralf G. Berger; Thin-layer high-vacuum distillation to isolate volatile flavour compounds of cocoa powder. European Food Research and Technology 2006, 223, 675-681, 10.1007/s00217-006-0252-x.
- Li, Y.; Feng, Y.; Zhu, S.; Luo, C.; Ma, J.; Zhong, F. The effect of alkalization on the bioactive and flavor related components in commercial cocoa powder. J. Food Compos. Anal. 2012, 25, 17–23.
- Ramli, N.; Yatim, A.; Hok, H. HPLC Determination of Methylxanthines and Polyphenols Levels In Cocoa and Chocolate Products. Mal. J. Anal. Sci. 2001, 7, 377–386.
- Lang, R.; Mueller, C.; Hofmann, T. Development of a stable isotope dilution analysis with liquid chromatography-tandem mass spectrometry detection for the quantitative analysis of di- and trihydroxybenzenes in foods and model systems. J. Agric. Food Chem. 2006, 54, 5755–5762.
- Miller, K.B.; Hurst, W.J.; Payne, M.J.; Stuart, D.A.; Apgar, J.; Sweigart, D.S.; Ou, B. Impact of alkalization on the antioxidant and flavanol content of commercial cocoa powders. J. Agric. Food Chem. 2008, 56, 8527–8533.
- Rocha, I.S.; de Santana, L.R.R.; Soares, S.E.; Bispo, E.S. Effect of the roasting temperature and time of cocoa beans on the sensory characteristics and acceptability of chocolate. Food Sci. Technol. 2017, 37, 522–530
- Giacometti, J.; Jolić, S.M.; Josić, D. Chapter 73—Cocoa Processing and Impact on Composition. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 605–612.
- Jolic, S.; Redovniković, I.R.; Marković, K.; Šipušić, D.I.; Delonga, K. Changes of phenolic compounds and antioxidant capacity in cocoa beans processing. Int. J. Food Sci. Technol. 2011, 46, 1793–1800.
- Wollgast, J.; Anklam, E. Review on polyphenols in theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000, 33, 423–447.
- W Jeffrey Hurst; Susann H Krake; Stephen C Bergmeier; Mark J Payne; Kenneth B Miller; David A Stuart; Impact of fermentation, drying, roasting and Dutch processing on flavan-3-ol stereochemistry in cacao beans and cocoa ingredients. Chemistry Central Journal 2011, 5, 53-53, 10.1186/1752-153x-5-53.
- Midori Natsume; Naomi Osakabe; Megumi Yamagishi; Toshio Takizawa; Tetsuo Nakamura; Haruka Miyatake; Tsutomu Hatano; Takashi Yoshida; Analyses of Polyphenols in Cacao Liquor, Cocoa, and Chocolate by Normal-Phase and Reversed-Phase HPLC. Bioscience, Biotechnology, and Biochemistry 2000, 64, 2581-2587, 10.1271/bbb.64.2581.
- Ilja C. W. Arts; Betty Van De Putte; Peter C. H. Hollman; Catechin Contents of Foods Commonly Consumed in The Netherlands. 1. Fruits, Vegetables, Staple Foods, and Processed Foods. Journal of Agricultural and Food Chemistry 2000, 48, 1746-1751, 10.1021/jf000025h.
