Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Florence Geneste and Version 3 by Conner Chen.
Iodinated X-ray contrast media (ICM) as emerging micropollutants have attracted considerable attention due to their high detected concentration in water systems. It results in environmental issues partly due to the formation of toxic by-products during the disinfection process in water treatment.
- iodinated X-ray contrast media
- depollution
- byproducts
1. Introduction
The continuous increase in the human population has created a comparable growth in the demand for water resources. In such an urgent situation, protecting our aquatic environment to achieve a comprehensive sustainable development strategy is one of the most essential environmental topics of the past decades. It has been shown that abounding compounds can enter the environment, such as pharmaceuticals [1][2][3][1,2,3], disinfectants, and other contaminants [4][5][6][4,5,6]. One of the most important factors contributing to this phenomenon is hospitals’ effluents as a source of contaminants discharge, such as specific iodinated X-ray contrast media (ICM) [7][8][9][7,8,9], antitumor agents [10][11][10,11] as well as antibiotics [12][13][12,13].
Since the discovery of X-ray radiation by Wilhelm C. Rogentgen in 1895, the diagnostic imaging procedure has become a routine part of medicine due to the possible external visualization of the internal anatomic structures of a patient without surgery [14]. Suspensions of barium sulfate have been applied for radiographic imaging since 1910. However, the marked radiodensity and poor intrinsic affinity for the gastrointestinal mucosa of this agent led to inaccurate diagnoses. In such a background, water-soluble iodinated X-ray contrast media (ICM) are the most extensively used pharmaceuticals for intravascular administration [15].
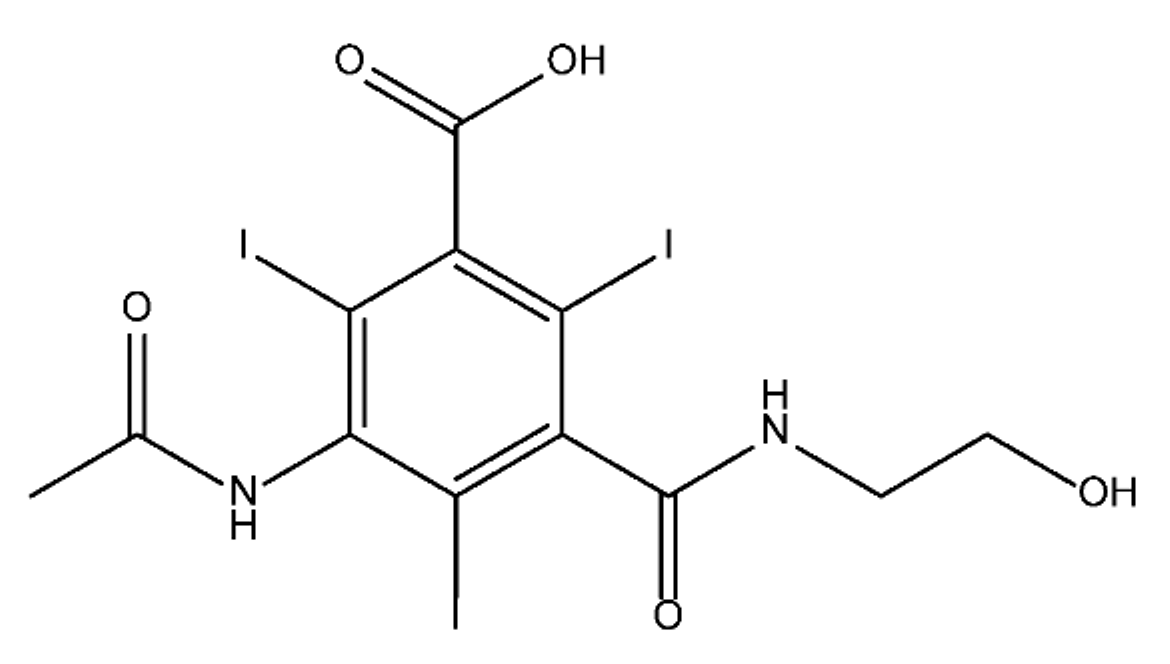
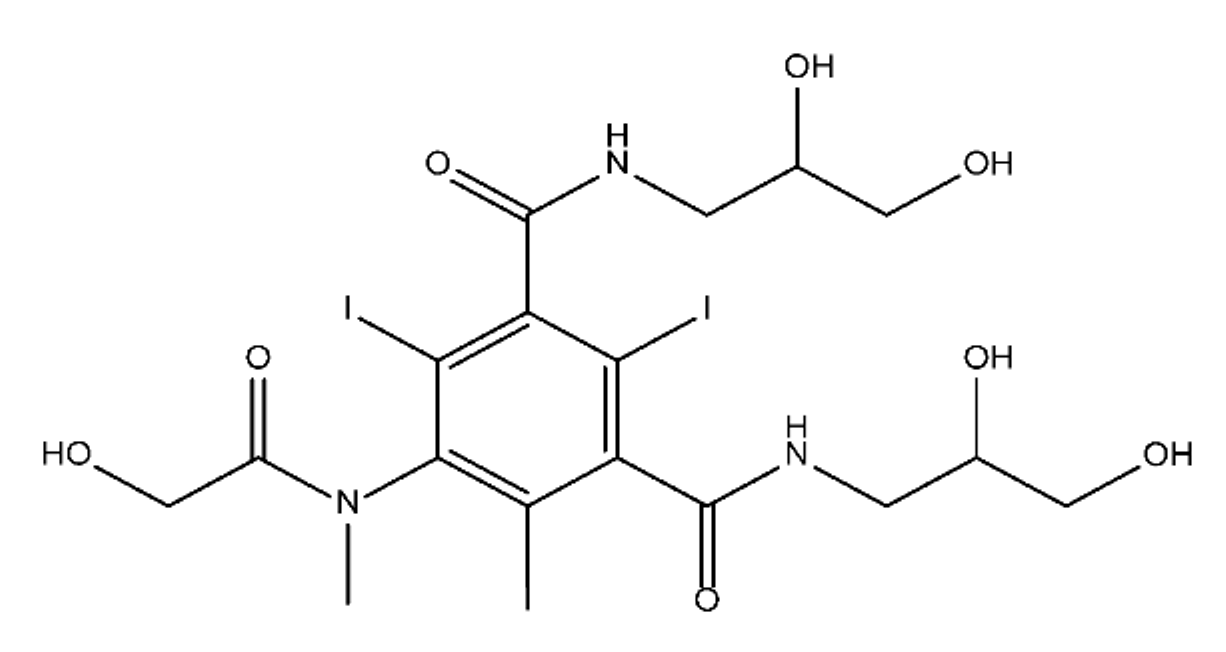
ICM are derivatives of 2,4,6-triiodobenzoic acid with polar carboxyl and hydroxyl moieties in their chains. Some of them are ionic, i.e. have one or several free carboxyl groups, and others are amide derivatives. They have all three iodine atoms in their chemical structure, which are responsible for the absorption of X-rays.
As ICM have been widely used for more than 40 years, their production is well-controlled. However, their synthesis is still the subject of high investigations to make it greener [16] and more efficient [17][18][17,18]. The recovery of the iodine atoms in industrial wastewater is also well-studied as it can reduce the cost of the overall production process [19][20][19,20].
2. ICM in the Environment
2.1. Effluents Polluted by ICM
From the internet site of the French National College of Medical Pharmacology (https://pharmacomedicale.org, accessed on 28 December 2022), 1.5 million contrast media injections were notified in France in 2018 and the most used were iodinated contrast media. Moreover, in the nineties, the annual consumption of ICM worldwide is estimated to have reached a value of 3.5 × 106 kg, since they are administered intravascularly in very high doses (up to 200 g ICM, corresponding to approximately 100 g iodine per application) [21][23]. In addition, ICM has an average half-life in the human body of around 2 h, and 75% of the administered dose is excreted via urine or feces into the hospital wastewater within 4h and nearly 100% within 24 h [22][24]. They were identified as a major source of increased absorbable organic halogen (AOX) concentrations in hospital sewage water [23][25]. In a large part of the region, hospital wastewater is not treated separately and is finally discharged into the local sewage systems together with domestic wastewater. Even if wastewater treatment plants (WWTP) can eliminate most pharmaceutical chemicals during the treatment process, various treatment systems are required for different contaminants in order to achieve efficient removal. Pollutants with polar characters can easily bypass the water purification process and so reach surface waters with a potential impact on the ecosystem and human health [24][26]. The n-octanol/water partition coefficient of ICM (Table 1) characterizes them as very hydrophilic.Table 1.
Chemical structure and physicochemical properties of ICM.
| Diatrizoic Acid | Iohexol | Iopamidol | Iopromide | Ioxithalamic Acid | Iomeprol | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structure | 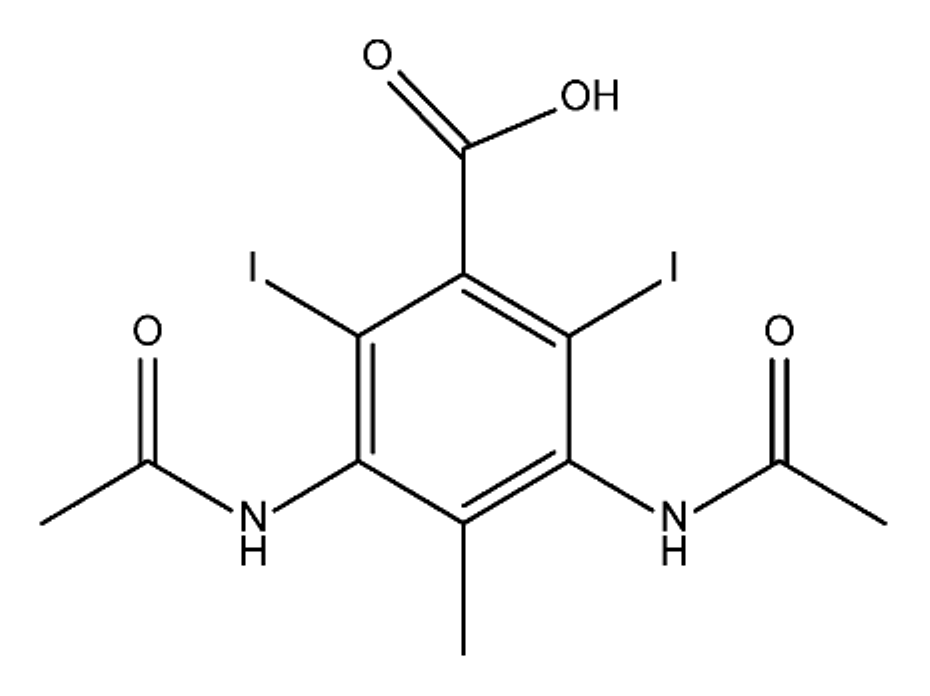 |
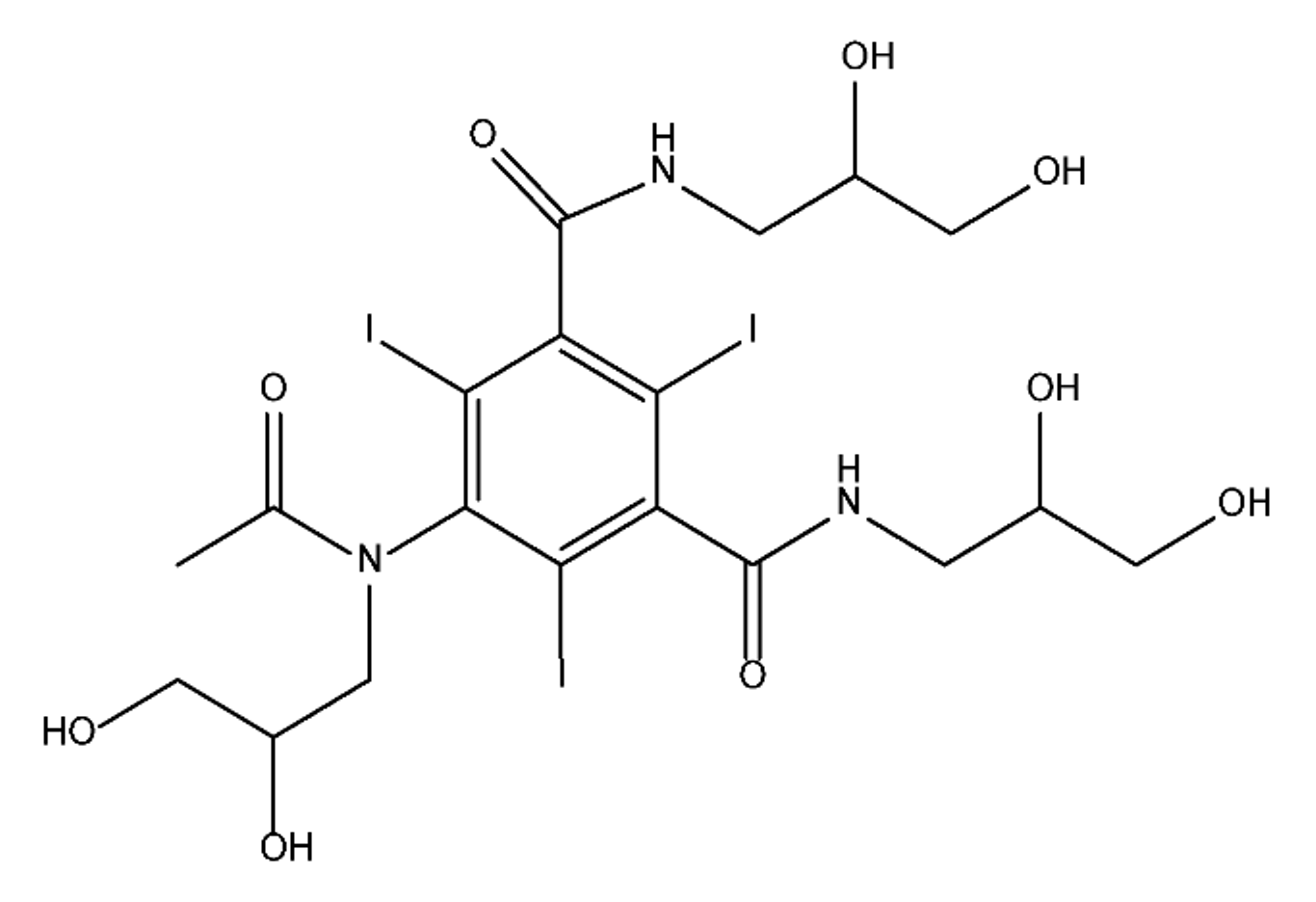 |
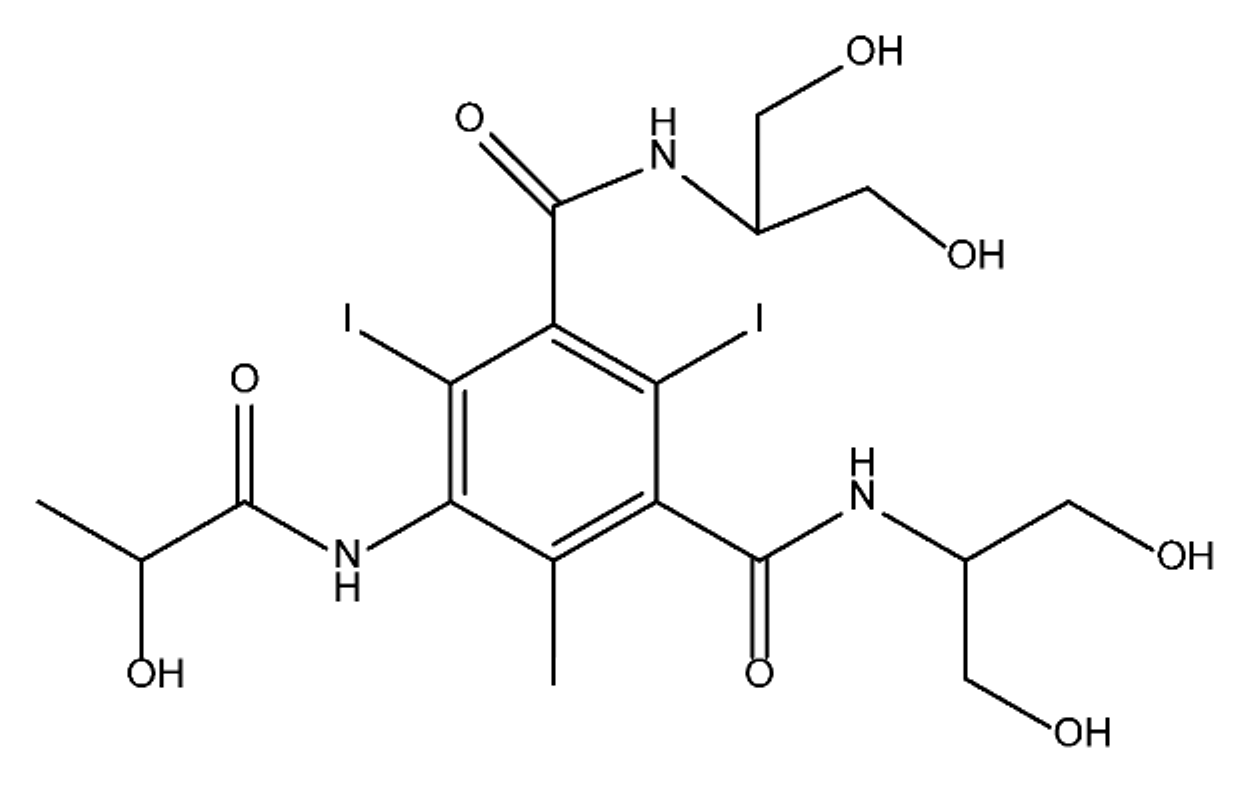 |
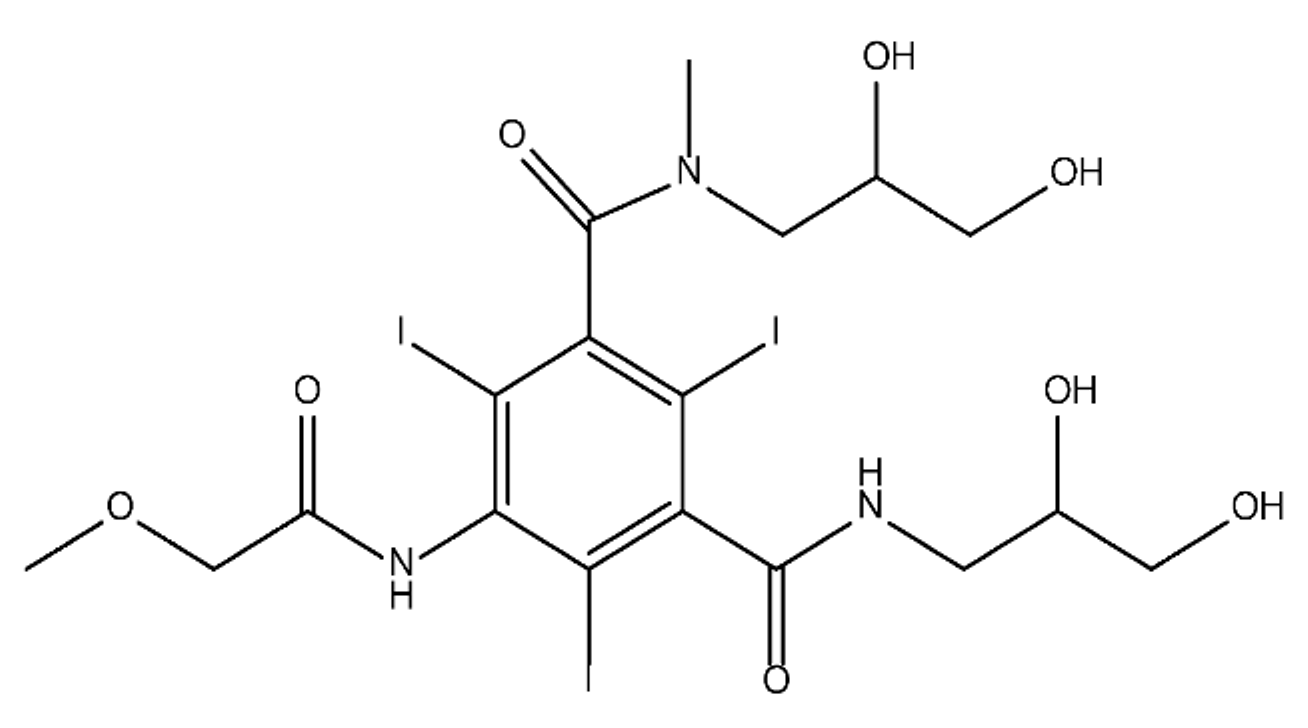 |
 |
 |
||||||||||||||||||||||||||||
| Log K | ow | a | 1.8 | −3 | −2.4 | −2.1 | 1.2 | −2.3 | ||||||||||||||||||||||||||
| Ionicity | Ionic | Nonionic | Nonionic | Nonionic | Ionic | Nonionic | ||||||||||||||||||||||||||||
| pKa | 2.17 | 11.73 | 11.00 | 11.09 | 2.13 | 11.73 | ||||||||||||||||||||||||||||
| Concentrations | b | (μg L | −1 | ) | ||||||||||||||||||||||||||||||
| Hospital wastewater | 17.1–61 | 0.07–3810 | 0.03–2599 | 0.008–3.2 | 15–550 | 0.05–2400 | ||||||||||||||||||||||||||||
| Surface water | 0.032–4.55 | 0.01–1.326 | 0.008–3.2 | 0.01–13 | 0.01–0.438 | 0.023–6.1 | ||||||||||||||||||||||||||||
| Ground water | 0.02–9.6 | 0.003–0.187 | 0.006–0.47 | 0.003–0.687 | 0.204 | 0.003–1.655 | ||||||||||||||||||||||||||||
| Drinking water | 0.0009–1.2 | 0.001–0.034 | 0.02–0.27 | 0.0005–0.084 | - | 0.0013–0.034 | ||||||||||||||||||||||||||||
