Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Carmine Izzo and Version 2 by Catherine Yang.
Cardiovascular diseases (CVDs) are the leading cause of death and illness in Europe and worldwide, responsible for a staggering 47% of deaths in Europe. There has been increasing evidence pointing to bioactive sphingolipids as drivers of CVDs. Among them, most studies place emphasis on the cardiovascular effect of ceramides and sphingosine-1-phosphate (S1P), reporting correlation between their aberrant expression and CVD risk factors.
- sphingolipids
- cardiovascular diseases
- cerebrovascular diseases
1. Introduction
Sphingolipids, including sphingosine, ceramide, sphingosine-1-phosphate (S1P) and ceramide-1-phosphate, are bioactive components in cell membranes that participate in and regulate numerous biological processes, such as cell proliferation and survival, maturation, senescence and apoptosis [1][2][3][1,2,3]. Sphingolipids can be synthesized via the de novo synthesis pathway, but they can also be formed through the sphingomyelinase pathway and/or the so-called “salvage” pathway (Figure 1).
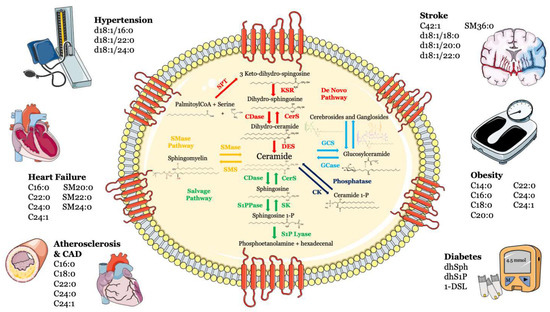
Figure 1. Metabolism and structure of sphingolipids and their implication in cardio- and cerebrovascular diseases. Sphingolipid metabolism and structure are illustrated inside the cell. Ceramide is the heart of the sphingolipid metabolic pathway. Ceramide can be synthesized through several steps: (i) de novo synthesis pathway starting from l-serine and palmitoyl-CoA (red); (ii) SMase pathway, through hydrolysis of sphingomyelin (yellow); (iii) salvage pathway, long-chain sphingoid bases are reused to form ceramide through the action of ceramide synthase (green); (iv) or through hydrolysis of glycosphingolipids and sulfatites (azure). Ceramide can also be synthesized from ceramide-1-phosphate through the action of ceramide-1-phosphate phosphatase (blue). The main cardiovascular diseases and risk factors in which sphingolipids may be used as biomarkers are summarized outside the cell. Abbreviations: serine palmitoyl-CoA-acyltransferase (SPT), 3-ketosphinganine reductase (KSR), (dihydro)-ceramide synthase (CerS), ceramide desaturase (DES), ceramide kinase (CK), glucosylceramide synthase (GCS), glucosyl ceramidase (GCase), ceramidase (CDase), sphingosine-1-phosphate lyase (S1P lyase), sphingosine kinase (SK), sphingosine 1-phosphate phosphatase (S1PPase), sphingomyelin (SM) synthase (SMS), sphingomyelinase (SMase).
Over the last decade, growing numbers of studies have highlighted the role of sphingolipids in the pathogenesis of CVDs [4][5][6][7][8][9][4,5,6,7,8,9]. In mice and rats, repression of sphingolipid biosynthesis attenuates cardiometabolic risk factors, including glucose intolerance, insulin resistance, diabetes, hypertension, atherosclerotic plaque development, arterial dysfunction and heart failure (HF) [10][11][12][13][14][10,11,12,13,14]. Data from patients have also indicated associations of tissue and circulating levels of sphingolipids with increased risk of CVDs, including HF, hypertension, metabolic syndrome and coronary artery disease (CAD) [12][15][16][17][18][12,15,16,17,18].
2. Atherosclerosis and Coronary Artery Disease
Sphingolipids, and in particular, ceramide, can contribute to the pathogenesis of atherosclerosis [19], an inflammatory and potentially lethal condition characterized by the generation of atheromatous plaques, consisting of cholesterol and other lipids [20], in medium- and large-sized arteries [21]. Ceramide can be generated from sphingomyelin (SM) via the activation of the de novo synthesis pathway or from sphingomyelinases (SMases). The development of an atherosclerotic lesion involves a large number of proinflammatory cytokines, such as tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β) [22], which, along with oxidized low-density lipoprotein (oxLDL), can stimulate ceramide generation via sphingomyelin hydrolysis [23]. In their work, Laulederkind and colleagues [24] demonstrated that C2-ceramid is able to induce interleukin 6 (IL-6) gene expression in human fibroblasts, a cytokine known to be involved in inflammation [25] and for its ability to induce the liver’s production of the greatest predictor of future cardiovascular risk that has direct proinflammatory effects: C-reactive protein [26]. It has also been proven that oxLDL stimulates an enzymatic cascade that includes neutral SMase, ceramidase and sphingosine kinase, thereby promoting the production of S1P to stimulate mitogenesis [27] and proliferation of smooth muscle cells (SMCs) [28][29][28,29], a hallmark of atherosclerotic lesion development. Moreover, Li and collaborators [30] showed that endogenous ceramides contribute to the subendothelial infiltration of oxLDL into the vessel wall.
Deficiency in or pharmacologic inhibition of neutral SMase2 in an Apolipoprotein E (ApoE)-null mouse model resulted in a decrease in atherosclerotic lesions and macrophage infiltration and lipid deposition via a mechanism involving the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway [31]. S1P is also released from activated platelets and interacts with endothelial cells during the atherosclerotic process [32]. It has also been proven that S1P can induce platelet shape change and aggregation [33] and that ceramide stimulates the release of the plasminogen activator inhibitor (PAI-1) [23][34][35][23,34,35], contributing, once again, to a variety of pathophysiological processes, such as thrombosis and atherosclerosis. In line, high endogenous S1P levels exaggerated atherosclerotic lesion development and increased plasma cholesterol levels in ApoE-null mice [36].
As mentioned above, tumor necrosis factor is able to activate a neutral sphingomyelinase [37], causing an increase in ceramide that, during the inflammation process, can act as an intermediate in TNF signaling in endothelial cells [38]. Furthermore, treatment of these cells with a water-soluble synthetic C8-ceramide or sphingomyelinase C led to the evidence that ceramide can induce NF-κB translocation to the nucleus and an increase in surface expression of E-selectin [38], which mediates the interaction of leukocytes with endothelium [39], thus, contributing to the initial stage of the disease. These sphingolipids can also evoke endothelial cell apoptosis [40][41][42][40,41,42], causing plaque erosion and unleashing other complications combined with the atherosclerotic process [23].
Another mechanism involved in the plaque formation induced by sphingolipids is represented by ceramide’s inclination to self-aggregate. It has a primary role in atherosclerosis’s onset since it contributes to the accumulation of LDL rich in this sphingolipid [43][44][43,44]. This notion is also supported by the evidence that ceramide content in LDL present in the atheromatous plaques is significantly higher than the plasma LDL and this sphingolipid was found only in aggregated LDL [45]. Furthermore, ceramide and other sphingolipids’ levels were increased in human atherosclerotic plaques and associated with plaque inflammation and instability [46][47][46,47].
Several clinical trials have highlighted the role of sphingolipids in atherosclerosis, reporting increased plasma concentrations of ceramides, sphingomyelins, sphinganine and sphingosine in patients with CAD [48]. An interesting clinical trial through a large-scale metabolomic analysis on a total of 200 patients tried to identify potential biomarkers for early-stage atherosclerosis. Analyses showed increased levels of 24 metabolites and a decrease in another 18 metabolites. Overall, nine metabolites were found to be suitable as combinatorial biomarkers, with an acceptable diagnostic accuracy [49]. In patients with CAD, the new PCSK9 has also been shown to significantly alter plasma lipidome composition and not only lipoprotein particles (LDL-C). Although the target for CVD prevention remains the lowering of LDL-C, the lipidome represents an asset for cardiovascular risk prediction and modification [50]. Similarly, treatment with fenofibrate showed not only the expected decrease in triglycerides, LDL-C and total cholesterol, but also an independent reduction in ceramide levels and of plasma apoC-II, apoC-III, apoB100 and SMase, with an increase in apoA-II and adiponectin levels [51]. A bi-ethnic angiographic case-control study showed increased plasma levels of SM in patients with coronary artery disease [48]. This was observed in both African-American and white participants, with a multivariate logistic regression analysis independent of other cardiovascular risk factors [48]. Using an unbiased machine learning approach, Poss et al. [52] identified 30 sphingolipids that were significantly elevated in the serum of patients with CAD (n = 462) compared with healthy controls (n = 212). Circulating ceramides were strongly correlated with disease severity since their levels were higher in subjects with CAD severity and major adverse cardia and cerebrovascular events (MACEs) [52][53][54][55][52,53,54,55]. Moreover, elevated levels of specific ceramide species (C16:0, C18:0, C22:0, C24:0 and C24:1) were associated with increased thrombotic risk, adverse CAD incidents and all-cause mortality [8][56][57][58][59][60][8,56,57,58,59,60]. Based on these findings, the authors of the study suggest serum ceramides as powerful biomarkers of CAD that could be useful to improve risk stratification [52].
