Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Wael Hegazy and Version 2 by Rita Xu.
Urinary tract infections (UTIs) represent one of the most common infections that are frequently encountered in health care facilities. One of the main mechanisms used by bacteria that allows them to survive hostile environments is biofilm formation. Biofilms are closed bacterial communities that offer protection and safe hiding, allowing bacteria to evade host defenses and hide from the reach of antibiotics. Inside biofilm communities, bacteria show an increased rate of horizontal gene transfer and exchange of resistance and virulence genes.
- recurrent urinary tract infections
- catheter-associated urinary tract infections
- biofilm formation
1. Introduction
Urinary tract infections (UTIs) are one of the most common bacterial infections in humans, and account for 40% of all nosocomial infections [1][2][3][4][1,2,3,4]. The incidence of UTIs among the population has increased by 60% between the years 1990 and 2019, confirming that UTIs represent a common public health problem that is far from being eradicated [5]. Typical symptoms include bacteriuria accompanied by urinary frequency, urgency, dysuria and suprapubic discomfort [6]. UTIs can be classified into complicated and uncomplicated urinary tract infections. Uncomplicated UTIs, commonly called cystitis, are a lower urinary tract infection, which only involves the bladder. Such infections are simple and easily resolved with antibiotic treatments. On the other hand, a complicated urinary tract infection is a simple infection that has progressed to the upper urinary tract and is usually associated with other risk factors. Complicated UTIs usually require longer antibiotic courses and are associated with high rates of treatment failure, recurrent infection, sepsis, and significant morbidity and mortality [6][7][8][6,7,8]. Uncomplicated UTIs are common in females, affecting 40–60% of females at least once in their lifetime, while all urinary tract infections in males are considered complicated [6][8][6,8]. Recurrent UTIs are defined as at least two acute episodes of UTIs occurring within a 6-month period or three episodes in a 12-month period, with much higher incidence in females than males [9][10][9,10]. The infection can be community-acquired, hospital-acquired or self-infected [10][11][12][10,11,12]. Most community-acquired infections result from poor personal hygiene, low sanitary precautions, or multiple sexual partners. Self-infection usually arises in immunocompromised individuals or high-risk cases, usually caused by commensal inhabitants from the periurethral, vaginal or rectal flora [13][14][13,14].
Biofilm formation represents the cornerstone in the pathogenesis of UTIs, since bacterial biofilms play a pivotal role in catheter-associated UTIs (CAUTIs), which account for 40% of all nosocomial infections [15][16][17][15,16,17]. Furthermore, biofilm formation is considered one of the most important mechanisms behind the high rates of recurrence and antimicrobial resistance that are often associated with UTIs [15][17][18][19][15,17,18,19]. Bacterial pathogens, both Gram-negative and Gram-positive, are the most common suspects behind UTIs [9][19][20][9,19,20]. Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Staphylococcus epidermidis, Staphylococcus saprophyticus, Staphylococcus aureus and Enterococcus faecalis are among the most frequent bacterial pathogens that are common in uncomplicated UTIs and complicated UTIs, as well as in CAUTIs [5][6][14][21][22][23][5,6,14,21,22,23]. This is not to mention the increasing role of Pseudomonas aeruginosa and Acinetobacter baumannii in complicated nosocomial UTIs [24][25][24,25]. However, some fungi, such as Candida spp., are also associated with high incidence of UTIs. Although using different pathogenic pathways, Candida spp. share bacterial pathogen with their ability to form persistent biofilms that contribute to chronic recurrent UTIs [11][26][27][28][11,26,27,28].
2. Urinary Tract Infections (UTIs)
2.1. Risk Factors
UTIs are very common infections, and one of the most common infections encountered in health care facilities. However, some risk factors are associated with high infection rates. The female gender represents an important risk factor; it is estimated that 40–60% of females will get a urinary tract infection at least once in their life, and half of them will experience recurrence within one year [8][9][10][8,9,10]. On the other hand, in males, the incidence of contracting UTI drops to 10–15%, and drops even further for circumcised males [6]. The risk of infection is higher in females due to many factors. First of all, females have a shorter urethra than males, which means a shorter distance that bacteria have to travel to reach the urinary bladder and initiate the infection [29]. Additionally, the incidence of self-infection from the perineal flora is higher in females, due to the anatomical differences between the two genders [8]. Hormonal fluctuations during menopause represent another female-related factor due to the drop in estrogen level, which makes urogenital skin thinner and reduces colonization by Lactobacilli. Colonization of Lactobacilli provides a protective barrier against pathogenic infection mediated by lactic acid production, which reduces vaginal pH, hence rendering the media less suitable for bacterial growth [30]. Pregnancy is another risk factor, due to reduced immunity [31]. Likewise, immunocompromised individuals and geriatrics are at particularly high risk [32][33][32,33]. Diabetes mellitus is one of the major risk factors contributing to high rates of complicated urinary tract infections, especially in catheterized cases [5][21][5,21]. This can be explained due to nephropathy, glucosuria and reduced immunity, which are common complications found in most diabetic patients [17]. Other risk factors include structural abnormalities of the urinary tract, renal stones, physical interventions like the use of catheters, spermicides, contraceptive diaphragms, intrauterine devices, and frequent pelvic examinations [6][14][6,14].2.2. Microbial Etiology of UTIs
Most urinary tract infections would result from self-infection; others are transferred from sexual partners, bad hygiene habits or hospital-acquired. UTIs can be caused by a wide range of Gram-negative and Gram-positive bacteria as well as fungi (Figure 1) [6][8][11][23][25][6,8,11,23,25]. The source of infection mostly arises from the normal commensal inhabitants of the urogenital, rectal and vaginal flora, with intestinal microbiota acting as the main reservoir of infection [8][23][8,23]. E. coli is by far the most commonly isolated uropathogen, accounting for 75% of uncomplicated UTIs and 65% of complicated UTIs [34]. K. pneumonia is the second-most common uropathogen, causing 6–8% of all UTIs, while E. faecalis and C. albicans are major causative agents of complicated UTIs, accounting for 11% and 7% of infections, respectively [14]. Proteus mirabilis, Staphylococcus saprophyticus, Staphylococcus epidermidis, Staphylococcus aureus, group B Streptococcus, Pseudomonas aeruginosa and Acinetobacter baumannii are less frequently isolated from UTIs [35].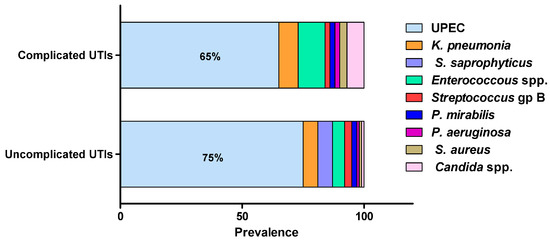
Figure 1. The most frequent uropathogens (adopted from Flores-Mireles, et al., 2015). UTIs are caused by a wide range of Gram-negative and Gram-positive bacterial pathogens as well as fungi. Uropathogenic E. coli (UPEC) is the most common causative agent for both uncomplicated (75%) and complicated (65%) UTIs. The most prevalent pathogens in uncomplicated UTIs, in order of prevalence, are Klebsiella pneumoniae (6%), Staphylococcus saprophyticus (6%), Enterococcus faecalis (5%), group B Streptococcus (3%), Proteus mirabilis (2%), Pseudomonas aeruginosa (1%), Staphylococcus aureus (1%), and Candida spp. (1%). For complicated UTIs, the most prevalent pathogens are Enterococcus spp. (11%), K. pneumoniae (8%), Candida spp. (7%), S. aureus (3%), P. mirabilis (2%), P. aeruginosa (2%) and group B Streptococcus (2%).
2.3. Pathogenesis of UTIs
The infection starts by the ability of some bacteria to cross the urethral sphincter muscle, which acts as a natural barrier against pathogens, followed by adherence to the urethral epithelium [19]. Adhesion is mediated by bacterial fimbriae and adhesins, which play a pivotal role in the initiation of urinary tract infections. Later, most pathogens would climb up the urethra by swimming to the urinary bladder [14][22][14,22]. Colonization in the urinary bladder is attained by simultaneous expression of multiple virulence factors – mainly motility, adhesion and secretion of toxins like proteases, hemolysins, urease, colony-necrotizing factors, siderophores, and polysaccharide coatings. These toxins would reflect tissue necrosis and facilitate invasion, giving rise to a typical cystitis [23]. Common symptoms are bacteriuria accompanied by fever, dysuria, pyuria, itching, urinary frequency, urgency lower abdominal pain (LAP), suprapubic pain, burning sensation during urination, blisters and ulcers in the urogenital area [7][36][7,36].2.4. Catheter-Associated Urinary Tract Infections (CAUTIs)
Urinary catheters are tubular devices made of latex or silicone that are widely used in hospitalized patients. Catheter-associated urinary tract infections (CAUTIs) are the most common nosocomial infections, accounting for approximately 40% of all hospital-acquired infections [5][21][5,21]. Catheters are foreign bodies that induce local mechanical stress which in turn activates a wide range of inflammatory responses at the adjacent tissues such as exfoliation, oedema, and mucosal lesions, in addition to localized deposition of serum fibrinogen on the catheter surface [37]. Fibrinogen acts as a lubricating layer with the aim of reducing abrasion, but also provides an ideal niche for the attachment of pathogens and as a nutrient source [22]. Urinary catheters can be contaminated during insertion due to inadequate application of aseptic techniques; additionally, long-term catheterization (more than seven days) represents a significant risk factor for development of urinary tract infections, since the surface of urinary catheters acts as an attractive niche for bacterial adhesion and proliferation [17].3. Bacterial Biofilms
Bacterial biofilms are closed bacterial communities in which the bacteria are embedded in an extracellular polymer matrix secreted by bacteria itself. This viscous medium anchors the bacterial colony at the infection site and provides a protective shield against host defenses, as well as antimicrobial treatments [13]. Bacterial biofilm formation represents an important virulence factor in most uropathogens, playing an important role in infection persistence and recurrence [19]. Additionally, bacterial biofilms represent the main cause behind CAUTI, which is the most common complication in long-term hospitalized patients [38]. The bacterial population within the biofilm shows an altered behavior to that of planktonic bacteria; bacteria rearrange their priorities in order to fortify their attack plan, where motility and metabolic activities are reduced in order to conserve energy and nutrients [19][39][19,39]. At the same time, extracellular toxins are upregulated in order to reflect maximum possible tissue damage; this ensures generous release of nutrients at the infection site and further cements the biofilm in site [40][41][40,41]. Meanwhile, the biofilm community would continue to shed daughter planktonic cells that aim to seed adjacent tissues, causing the spread of infection and development of new biofilms [13]. Eventually, a bacterial biofilm would develop into a mature, densely-packed structure that is very difficult to eradicate, hence contributing to chronic and recurrent infections [19].3.1. Biofilm Formation Mechanism
The stages involved in biofilm formation have been extensively studied in order to identify possible mechanisms for biofilm inhibition (Figure 2) [13][28][40][41][42][43][44][13,28,40,41,42,43,44]. The first stage in biofilm formation is the reversible attachment of freely-swimming planktonic bacteria to a suitable surface, which can be found in a wide variety of sites or niches. Inanimate hydrophobic surfaces like polystyrene catheters, prosthetic devices, stents or rough surfaces like renal stones represent an ideal surface for biofilm attachment. This step is driven by different forces like hydrophobic interactions, Van der Waals forces, or electrostatic attraction. The attachment can be easily affected by media pH, temperature and solutes [42][45][46][42,45,46]. At some point, attachment becomes irreversible due to the stronger forces of adhesion mediated by bacterial adhesins. Noteworthy is the fact that inhibition of this step (by downregulation of pili formation or by adhesins antibodies) can drastically diminish biofilm formation [47]. The next stage is early development, in which the attached bacteria start replicating at site, causing an increase in population number, which triggers the initiation of QS communication systems; this cell-to-cell communication will result in increased secretion of extracellular polysaccharides. After this is the biofilm maturation stage, in which biofilm biomass grows into a three-dimensional macro-colony in which the dominant bacterial phenotype is the persister cells. At this stage, biofilm eradication is very difficult [48]. Finally, at the dispersion stage, the biofilm starts shedding daughter planktonic cells that seed adjacent sites for the formation of new biofilms [49][50][49,50].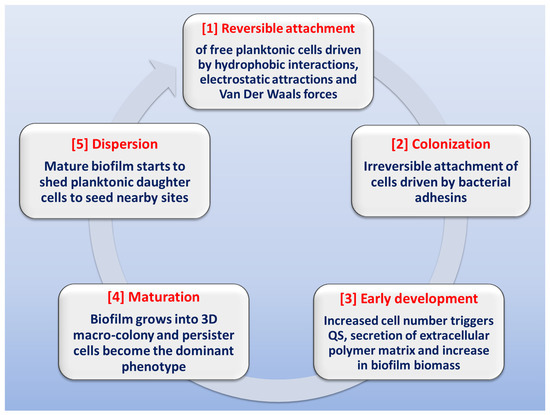
Figure 2. Schematic representation of the stages involved in biofilm formation.
3.2. Bacterial Communication within Biofilms
Quorum sensing (QS) is a bacterial communication system which allows the bacteria to coordinate their behavior using chemical molecules as a communication signal. It is important to understand that QS communication system aims at orchestrating a grouped bacterial behavior which results in maximum benefits for the population within the biofilm including optimum use of nutrients, increased pathogenicity, and extension of survival rates [18][51][18,51]. That being said, it is logical to expect a certain threshold for activation of the QS system by reaching a minimum population level in order to trigger the onset of the system [41][45][41,45]. The mechanisms of QS and its role in controlling group bacterial behavior have been extensively studied, revealing that QS system utilizes an inducer/receptor mechanism to control bacterial gene expression [18][43][51][52][53][18,43,51,52,53]. The same system is used in both Gram-negative and Gram-positive bacteria. However, the signaling pathway is different. Gram-negative bacteria recruit a system based on N-acyl-homoserine lactones (AHLs) inducers that are synthesized via AHL synthase genes, such as different luxI genes, and sensed by their cognate receptors LuxR-type QS receptors [54][55][54,55]. The AHLs molecules are secreted in minute amounts that are undetected at low bacterial population. However, at high population levels, the amount of secreted AHLs is enough to bind to specific bacterial receptors, forming QS receptor-AHL complex. After hitting a certain threshold concentration, the QS receptor-AHL complexes control the transcription of genes involved in biofilm formation [56][57][58][59][60][56,57,58,59,60]. On a parallel basis, QS systems in Gram-positive bacteria employ autoinducing peptides (AIPs) that are detected by two-component membrane-bound signal transduction systems [55][61][62][63][55,61,62,63]. The different QS systems in Gram-negative and Gram-positive bacteria are represented in Figure 3. QS plays a crucial role in biofilm formation through controlling the production of extracellular polymer matrix, upregulation of bacterial virulence genes, increasing secretion of exoenzymes, siderophores, antibiotics and bioluminescence [50][64][50,64].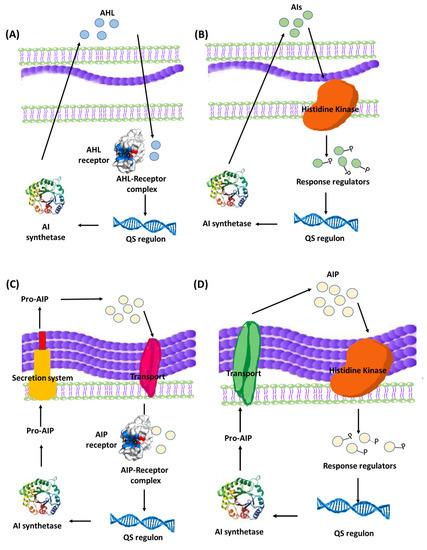
Figure 3. Bacterial QS systems in Gram-negative (A) two-component signaling, or (B) LuxI/LuxR-type QS systems. Gram-positive (C) two-component signaling, or (D) AIP-binding transcription factor QS systems. AI: Autoinducers, AIP: Autoinducing peptides.
4. Contribution of Biofilm Formation to Pathogenicity in Urinary Tract Infections
Bacterial biofilm formation represents an important virulence factor in uropathogens, playing an important role in infection persistence and recurrence [19]. Bacterial biofilms represent the main cause behind catheter-associated urinary tract infection, which is the most common complication in long-term hospitalized patients [18][39][42][18,39,42]. During a biofilm infection, simultaneous activation of both innate and acquired host immune responses may occur. While the free planktonic bacterial cells can be eradicated by the host immune response or antibiotics or a combination of both, the bacterial population within the biofilm remains highly protected and continues to reflect serious host tissue damages [16][18][16,18]. There are multiple mechanisms that make biofilms a hazardous complication that contributes to the pathogenicity and persistence of the infection.
4.1. The Extracellular Polymer Matrix
Consisting mainly of extracellular secreted polysaccharides (e.g., cellulose, polyglucosamine and alginates) in addition to a wide variety of proteins, glycoproteins, and glycolipids, extracellular DNA enzymes, signaling compounds, adhesins, nutrients, cell debris, wastes and surfactants [42][65][42,96]. The polymer matrix is highly hydrated, providing a viscous mucoid consistency that offers high adhesiveness to solid surfaces in addition to increased bacterial aggregation [19][66][19,97]. The overall result is increased biofilm biomass, increased viscosity, stronger attachment and bacterial proliferation within the biofilm [45]. The high viscosity of the biofilm prevents penetration of host immune defenses as well as antimicrobial treatments, thus contributing to the high resistance and persistence of biofilms [42].
4.2. Extracellular Toxins
Bacterial behavior within the biofilm is under quorum sensing control, resulting in upregulation of virulence factors that further fortify pathogenicity, so extracellular toxins are upregulated in order to reflect maximum possible tissue damage; this ensures generous release of nutrients at the infection site and further cements the biofilm in site [40][41][40,41].
4.3. Persister Cells
The limited availability of nutrients and oxygen within the biofilm results in sub-optimal bacterial growth rate and asynchronous microbial growth, giving rise to variant bacterial phenotypes within the biofilm. The most abundant phenotype is the persister cells that are known for their inherent resistance to antibiotics [18][38][39][65][67][68][69][70][18,38,39,96,98,99,100,101]. Persister cells are dormant, slow-growing cells with reduced metabolic activity and high tolerance to antibiotic treatment; these cells allow for fair distribution of nutrients, since their requirements are minimal. Additionally, they show altered metabolic pathways that result in loss of target site of most antibiotic treatments [48]. Moreover, the phenotypic variations among the biofilm-forming bacteria results in differential gene expression, giving rise to a cocktail of bacterial responses including toxin release, efflux systems activation, ion sequestering, and lipid biosynthesis, in addition to antibiotic resistance [45][50][45,50]. These different responses would serve as a rich pool for selection of phenotypes with the highest survival rates; eventually, natural selection would favor the most virulent and resistant bacteria that will dominate the bacterial population within the biofilm [41][50][71][72][41,50,102,103].
4.4. Horizontal Gene Transfer
The high bacterial density within the biofilm results in increased rate of horizontal gene transfer (HGT) between members of the biofilm community, which can take place between cells from the same or different species. This involves exchange of genetic elements such as transposons [73][104], plasmids [74][105], bacteriophages [75][106] and integrative conjugative elements (ICEs) [76][107]. There are three main mechanisms for HGT, depending on the type of transferred DNA: conjugation (for transfer of conjugative plasmids and ICE), transformation (for transfer of short pieces of chromosomal DNA and non-conjugative plasmids), and transduction (for bacteriophage mediated gene transfer) [75][77][78][106,108,109]. In addition to the three HGT mechanisms, membrane vesicles (MVs) have been recently documented as a cargo carrier that can mediate interspecies HGT, changing the expression of biofilm-related genes, and transferring antibiotic resistance genes [79][80][110,111]. The MVs can transfer DNA or sRNA that can encode diverse genes involved in virulence, stress response, and antibiotic resistance [80][111]. A schematic summary of the main mechanisms involved in HGT is presented in Figure 4.
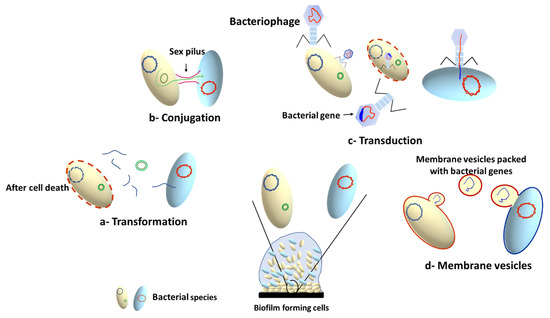

Figure 4. Horizontal gene transfer mechanisms in microbial biofilms.
4.5. Resistance of Biofilms to Antibiotics
The resilient resistance of biofilms to antibiotics is a serious complication that is commonly implicated in most recurrent UTIs. Biofilms show an increased resistance to antibiotics that is estimated to be 10 to 1000 times greater than that of planktonic cells [81][112]. The resistant nature of biofilms is attributed to the collective effects of multiple factors: (i) the extracellular polymer matrix which confers a physical protective barrier against antibiotic penetration, hence diminishing the ability of antibiotics to reach hidden bacteria within the biofilm [18][39][65][82][18,39,96,113]; (ii) secretion of antibiotic inactivating enzymes that are upregulated under quorum sensing control [67][98]; (iii) phenotypic variations within the biofilm and the formation of persister cells which are inherently resistant to antibiotics as a result of their suppressed metabolic activity; (iv) increased expression of efflux pumps, which are membrane-bound transport proteins involved in the expelling of toxic molecules outside of the bacterial cell, including antimicrobial molecules; and (v) horizontal gene transfer (HGT) of antibiotic resistance genes. The HGT examples and their role in antimicrobial resistance development in biofilms are widely reviewed in many previous studies [77][78][79][83][84][108,109,110,114,115]. Additionally, efflux pumps allow the microorganisms to organize their internal environment by removing wastes, metabolites and quorum sensing signal molecules [85][116]. Furthermore, the biofilm bacteria share virulence factors’ encoding genes that make them reveal physiological and morphological changes [18][26][74][86][18,26,105,117]. For instance, the depletion of nutrients and oxygen in the biofilms leads to sub-optimal bacterial growth rates and shift in the phenotypic variations towards the formation of the persister cells. Interestingly, biofilms may be formed as a defensive consequence to the presence of antibiotics; for instance, the persistence of antibiotics as aminoglycosides, tetracycline, or cephradine at sub-inhibitory concentrations induce E. coli and P. aeruginosa to form biofilms [82][87][113,118].
