Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Paige Matheson.
Arthropods—insects, arachnids, myriapods, and crustaceans—comprise the bulk diversity of species on Earth, with some taxa that have survived all five mass extinction events in evolutionary history (e.g., horseshoe crabs). Situated at opposite ends of the Earth, the polar regions (Arctic and Antarctic) are some of the most harsh, remote, and inhospitable habitats on the planet. All polar-dwelling arthropods are exposed to cold temperatures, often below the freezing point of their body fluids. Resident species, at least to some degree, have a variety of adaptations that allow them to tolerate these local conditions.
- adaptation
- Antarctic
- Arctic
1. Introduction
Climate change is having increasingly detrimental impacts on the natural environment. In particular, potential warming beyond 1.5 °C is predicted to increase extreme weather events (e.g., floods, droughts, cyclones), impact human livelihoods by limiting potable water/food availability and damaging infrastructure, and threaten many species with extinction due to physically intolerable conditions, changing the entire landscape of ecosystems [1].
Situated at opposite ends of the Earth, the polar regions (Arctic and Antarctic) are some of the most harsh, remote, and inhospitable habitats on the planet (Figure 1). Where the Arctic is an ocean covered by perennial sea ice and surrounded by land, Antarctica is a continent covered by thick ice and surrounded by the Southern Ocean. Both are considered ‘barometers’ of global health for the role they play in providing planetary-scale balance and circulation—regulating energy exchange between climatic and oceanic systems, driving atmospheric and weather processes, acting as sinks for carbon dioxide, and providing thermal density gradients that drive thermohaline circulation [2,3][2][3]. Observed and projected climatic changes in the Anthropocene are comparable to some of the largest environmental changes of the past 65 million years, though human activities such as fossil fuel combustion and land-use change have meant that this is occurring much more rapidly than previously observed in the geological record [4]. Despite their isolation from civilisation, the Arctic and Antarctic polar regions have responded rapidly to human-induced climate change, with small changes in temperature having large impacts on species diversity and distribution [5,6,7][5][6][7].
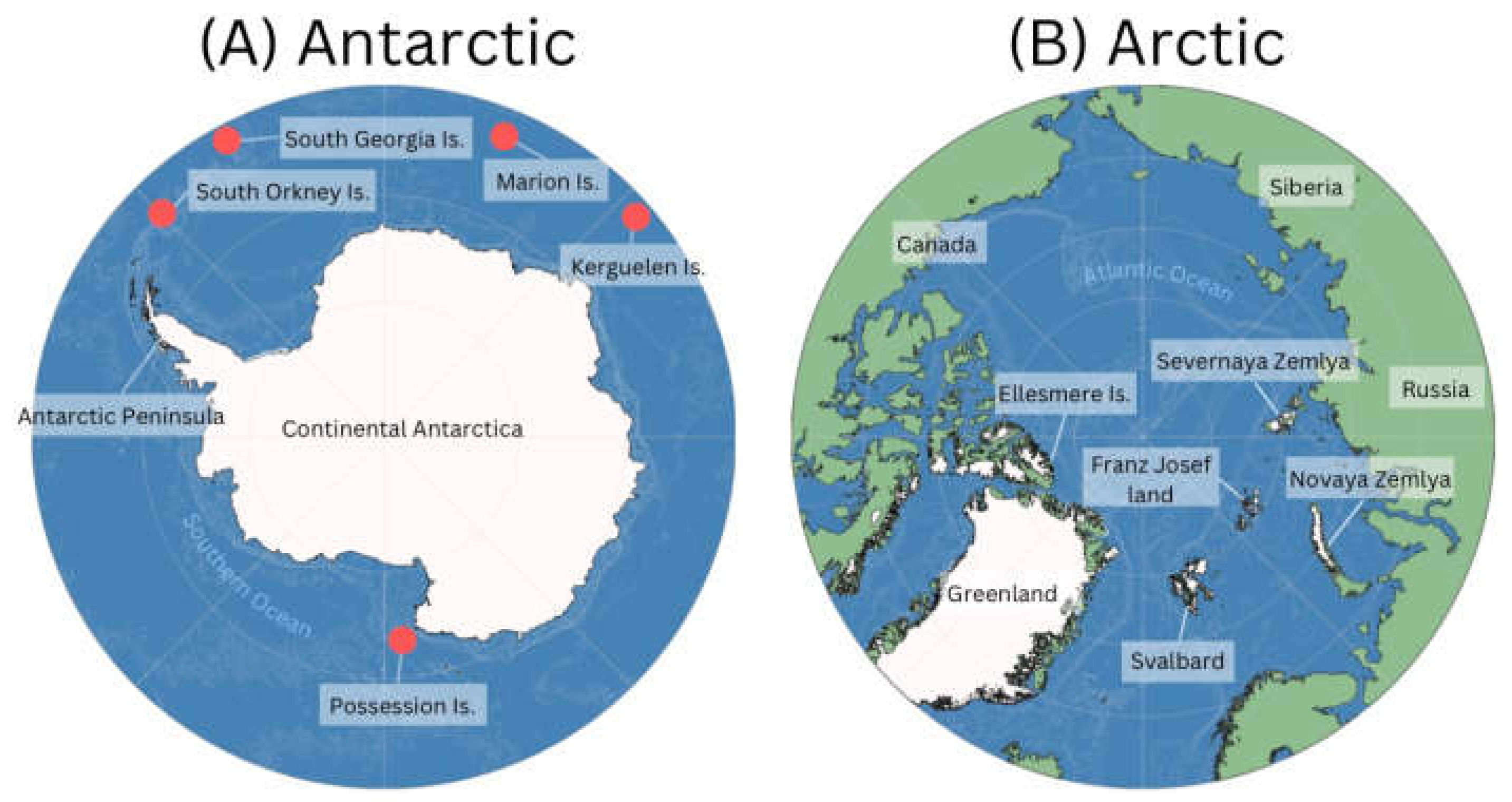
Figure 1. Map of (A) Antarctic and (B) Arctic regions identifying key locations. Red circles in (A) represent relative locations of the main sub-Antarctic Islands.
Arthropods—insects, arachnids, myriapods, and crustaceans—comprise the bulk diversity of species on Earth, with some taxa that have survived all five mass extinction events in evolutionary history (e.g., horseshoe crabs) [8]. As such, they are fundamental members of many ecosystems, playing crucial roles in food webs, pollination, decomposition, nutrient cycling, and pest control [9]. Polar regions are no exception, with some biota that have been present for millions of years throughout glacial cycles and since the breakup of Gondwana, and others that have arrived since the last glacial maximum (<30 kya) [10,11][10][11]. Resident polar arthropods must endure environmental stresses beyond the physiological limits of many other species, such as extremely low temperatures (routinely <−15 °C), intense heat stress due to solar radiation that exceeds air temperature (>20 °C) during summer months, dry and windy conditions, and 24 h of darkness during winter [12,13,14][12][13][14]. As a result, these taxa have evolved unique physiological adaptations to tolerate their local environment.
2. Polar Arthropods Are Abundant and Diverse, and Exhibit a Range of Adaptations to the Cold
2.1. Abundance and Diversity
The biodiversity of polar arthropods was once thought to be scarce owing to an overly simplistic understanding of their ecology and roles in the ecosystem. For instance, past Arctic food web models collated individual species into related groups (e.g., considered all beetle species one entity), neglecting to account for the wide range of feeding specialisations and adaptations that exist at the species level [15]. In reality, arthropods are the most diverse and abundant phylum found near the poles, accounting for approximately 90% of all known species there, though many are small and have only basic body forms [15,16][15][16]. In Antarctica, most terrestrial arthropods are restricted to the less harsh sub-Antarctic islands. For example, at least 41 endemic species of insects and spiders occupy Possession and Kerguelen Islands alone [17]. However, some species inhabit mainland Antarctica in high densities—including soil microarthropods (e.g., springtails, mites), most of which are endemic to the continent [18], and two species of chironomid midges (endemic Belgica antarctica and native Parochlus steineii). The majority of Arctic arthropod diversity is similarly distributed in less harsh environments of the sub-Arctic and low-Arctic regions, and predominantly consists of springtails, lice, chironomid midges, crane flies, aphids, beetles, moths, and wasps. In the high-Arctic, the same orders exist, though with much fewer species [15,19][15][19].2.2. Adaptations to the Cold
All polar-dwelling arthropods are exposed to cold temperatures, often below the freezing point of their body fluids. Resident species, at least to some degree, have a variety of adaptations that allow them to tolerate these local conditions, including physiological, plastic, behavioural, and genetic (Figure 2). For example, some microarthropods have developed parasitic relationships with warm-blooded hosts (e.g., ticks with seals or birds), while others buffer against immediate environmental temperatures by living in a microclimate (e.g., soil or water), or migrating seasonally [14]. By far the greatest focus of research to date has been on physiological adaptations of polar arthropods; we outline some of these below.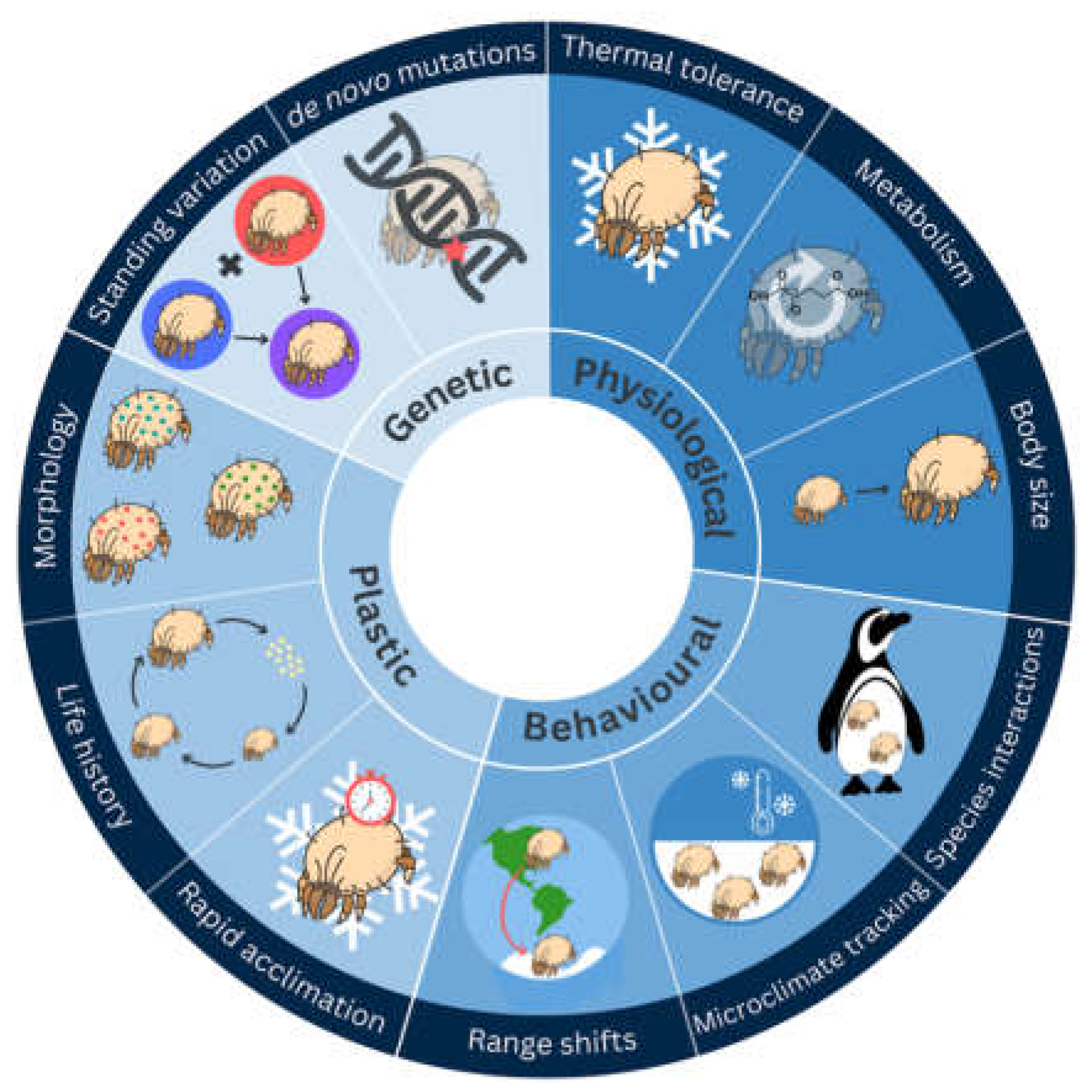
Figure 2.
The potential physiological, behavioural, plastic, and genetic responses associated with surviving life at the poles.
2.2.1. Thermal Tolerance
Thermal tolerance is the ability of an organism to survive short- or long-term exposure to temperature extremes. The ectothermic nature of arthropods means that temperature plays a major role in the effectiveness of their chemical and biological functions, therefore, thermal tolerance is a critically important trait for survival and success at the poles [20]. Polar arthropods can be classified as either freeze-avoidant or freeze-tolerant depending on their strategy to survive overwintering, although the factors that determine which species employ which strategy are largely unclear.Freeze-Avoidance
Most polar arthropods are freeze-avoidant—they survive subzero temperatures in a supercooled state by lowering the point at which their body fluids freeze, referred to as the super-cooling point (SCP) [18,21][18][21]. This is achieved through the accumulation of antifreeze and/or heat shock proteins [22], or by the removal of ice-nucleating particles, such as food and microbes in the digestive tract. Bimodal SCP distributions are commonly described, with less cold-hardy species being more active and higher-foraging, while more cold-hardy species are thought to be non-feeding and therefore lacking in ice nucleators from food in the gut [18,23][18][23]. However, this is not the case for all species. For example, the Antarctic springtail Gomphiocephalus hodgsoni prevents inoculative freezing of body fluids to temperatures as low as −35.4 °C through the accumulation of thermal hysteresis proteins and increased haemolymph osmolality [18,24,25][18][24][25].Freeze-Tolerance
Freeze-tolerant arthropods can withstand the formation of ice between their body tissues. They prevent intra-tissue damage by producing ice-nucleating and heat-shock proteins [26], and/or accumulating cryoprotectants (polyols and sugars) that control the rate of freezing and protect the membrane bilayer [27]. Freezing is often initiated slowly at relatively high subzero temperatures in order to promote the growth of small, site-specific ice crystals in the extracellular spaces, as opposed to the rapid formation of ice that can cause injury [22]. For example, an Arctic species of stonefly, Nemoura arctica, cools to around −1.5 °C before freezing and can then survive temperatures as low as −15 °C for over two weeks by increasing hemolymph glycerol concentrations and antifreeze proteins [28].Cryoprotective Dehydration
Cryoprotective dehydration (CPD) is a form of freeze-tolerance that occurs when an organism loses extreme amounts of body fluids to reach a vapour pressure equilibrium with surrounding ice. It has only been observed in a handful of arthropod species, including several Arctic springtails (e.g., Megaphorura arctica, Hypogastrura viatica, and Folsomia quadrioculata) [29[29][30],30], the Alaskan beetle Cucujus clavipes puniceus [31], and larvae of the Antarctic midge Belgica antarctica. In B. antarctica, ~40% of the body fluid is lost and high concentrations of solutes, such as osmolytes, accumulate until vapour pressure equilibrium with the surrounding ice is reached. This prevents internal freezing, allowing survival over 7–8 months of continuous subzero temperatures in a matrix of soil and ice [32].Rapid Cold Hardening
Polar environments provide increasingly variable microhabitat temperatures and unpredictable freeze–thaw periods in any season throughout the year. In response, some species have the ability to plastically enable cold tolerance via ‘rapid cold hardening’ (RCH)—a fast response to cold that can last a matter of minutes or hours [33]—providing an ecological advantage over species that must acclimate or adapt over much longer timescales. The mechanisms that underpin RCH are relatively unclear: it was thought that since RCH has similar ecological outcomes to seasonal freeze-tolerance or avoidance (i.e., higher survival rate, increased fitness at low temperatures), it is achieved through similar mechanisms albeit at shorter timescales. However, recent work suggests that RCH is rather mechanistically distinct to seasonal cold-hardening. For instance, cryoprotectant/antifreeze protein synthesis and up-regulated stress-related genes (i.e., heat shock proteins) are important factors associated with seasonal cold hardening, but either are not involved in, or act ambiguously during, RCH. Factors that are important to RCH include calcium signalling, inhibition of apoptotic cell death, membrane restructuring, and adjustments to ion transport mechanisms [34]. RCH has been identified in some native polar arthropods and microarthropods (e.g., Antarctic mites, Alakaozetes antarcticus and Halozete belgicae) [35] and is hypothesised to reduce the developmental cost of employing long-term overwintering strategies, such as freeze tolerance. However, others argue that RCH offers little advantage to some polar arthropods (such as the Arctic springtail, Hypogastrura tullbergi) that migrate through soil profiles during cold snaps, as this often offers sufficient protection from short-term low temperatures [36].2.2.2. Metabolism
While metabolism is mainly driven by the availability of food and oxygen, environmental temperatures have profound effects on metabolic rate, with higher metabolic rates across all phyla generally occurring in warmer climates and vice versa [37,38][37][38]. However, some research proposes that ectotherms from cold habitats have elevated metabolisms compared to those from warm habitats, owing to short, cool growing seasons that select for an increase in growth rates and development times—referred to as ‘metabolic cold adaptation’ [39,40,41,42][39][40][41][42]. Metabolic cold adaptation does not appear to be a phenomenon characterising all polar arthropods and warrants further research at the species level [43]. Nevertheless, metabolism produces the energy required for movement, growth, healing, feeding, digestion, and reproduction; thus, the ability to metabolise efficiently in cold environments may represent an ecologically advantageous trait for polar arthropods [44]. For example, during chill coma, arthropods lose ionic and osmotic homeostasis as the cellular membrane structure is altered and metabolic processes are down-regulated. As temperature increases again, whole-organism recovery involves restoring neuromuscular movement, repairing damage, and re-establishing ionic and osmotic gradients to restore homeostasis, all of which is energetically expensive. Thus, faster metabolism (and therefore recovery time) may allow for additional opportunities for foraging, dispersal, and reproduction, while reducing susceptibility to predation [39,45][39][45]. Indeed, arthropods from high-latitude environments may have a quicker chill coma recovery time than those from temperate regions [46]. Meanwhile, metabolomic studies have revealed abundant variation in core metabolic enzymes between polar species in response to thermal stress [12,47][12][47]. For example, B. antarctica enhances thermotolerance by increasing concentrations of metabolites, such as α-ketoglutarate (a Krebs cycle intermediate), to aid the synthesis of cryoprotectants [12]. In contrast, the Arctic seed bug, Nysius groenlandicus, actively alters metabolite levels on a daily basis, with higher concentrations of sugars found in individuals that were caught at the lowest daily field temperatures [48].2.2.3. Body Size
Body size is integral to an organisms’ ecological success—larger individuals tend to produce more offspring and live longer, and may be better at competing and avoiding predators than smaller individuals [49]. Body size is influenced by many factors, including metabolic energy, resource availability, and selection (both sexual and predation-driven) [50,51][50][51]. The temperature size rule (TSR) describes how individuals maintained at low temperatures tend to grow slower, but attain a larger body size upon maturity [52,53][52][53]. Similarly, Bergmann’s rule describes how larger individuals are found in higher latitudes and colder environments (i.e., higher altitudes) [52]. Body size distribution patterns have been found to be largely consistent with TSR and Bergmann’s rule for many endothermic species, such as birds and mammals [54]. Some ectothermic species follow these trends to a degree, though much less consistently, while other species follow a contradictory trend where body size decreases in response to cold temperatures (‘converse Bergmann’s rule’) [55]. For example, wing length in two high-Arctic butterfly species (Boloria chariclea and Colias hecla) decreased significantly between the years 1996 and 2013 in response to warmer Arctic temperatures, supporting TSR [52], whereas small insects characterised by rapid development (such as aphids and small flies) are dominant in both polar regions, in counter to Bergmann’s rule [56]. Variability in body size is likely to be idiosyncratic, depending largely on a number of species-specific and abiotic (e.g., oxygen) variables [49].References
- Pörtner, H.O.; Roberts, D.C.; Adams, H.; Adler, C.; Aldunce, P.; Ali, E.; Begum, R.A.; Betts, R.; Kerr, R.B.; Biesbroek, R.; et al. Climate change 2022: Impacts, adaptation and vulnerability. In IPCC Sixth Assessment Report; IPCC: Geneva, The Netherlands, 2022; pp. 37–118.
- Shadwick, E.H.; De Meo, O.A.; Schroeter, S.; Arroyo, M.C.; Martinson, D.G.; Ducklow, H. Sea ice suppression of CO2 outgassing in the West Antarctic Peninsula: Implications for the evolving southern ocean carbon sink. Geophys. Res. Lett. 2021, 48, e2020GL091835.
- Monteiro, M.R.; Marshall, A.J.; Hawes, I.; Lee, C.K.; McDonald, I.R.; Cary, S.C. Geochemically defined space-for-time transects successfully capture microbial dynamics along lacustrine chronosequences in a polar desert. Front. Microbiol. 2022, 12, 4201.
- Diffenbaugh, N.S.; Field, C.B. Changes in ecologically critical terrestrial climate conditions. Science 2013, 341, 486–492.
- Koenigk, T.; Key, J.; Vihma, T. Climate change in the Arctic. In Physics and Chemistry of the Arctic Atmosphere; Kokhanovsky, A., Tomasi, C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 673–705.
- Hughes, K.A.; Convey, P.; Turner, J. Developing resilience to climate change impacts in Antarctica: An evaluation of Antarctic Treaty System protected area policy. Environ. Sci. Policy 2021, 124, 12–22.
- Høye, T.T. Arthropods and climate change–Arctic challenges and opportunities. Curr. Opin. Insect Sci. 2020, 41, 40–45.
- Rudkin, D.M.; Young, G.A. Horseshoe crabs–an ancient ancestry revealed. In Biology and Conservation of Horseshoe Crabs; Tanacredi, J.T., Botton, M.L., Smith, D., Eds.; Springer: Boston, MA, USA, 2009; pp. 25–44.
- Høye, T.T.; Culler, L.E. Tundra arthropods provide key insights into ecological responses to environmental change. Polar Biol. 2018, 41, 1523–1529.
- Convey, P.; Gibson, J.A.; Hillenbrand, C.D.; Hodgson, D.A.; Pugh, P.J.; Smellie, J.L.; Stevens, M.I. Antarctic terrestrial life--challenging the history of the frozen continent? Biol. Rev. 2008, 83, 103–117.
- Hughes, K.A.; Worland, M.R. Spatial distribution, habitat preference and colonization status of two alien terrestrial invertebrate species in Antarctica. Antarct. Sci. 2010, 22, 221–231.
- Michaud, M.R.; Benoit, J.B.; Lopez-Martinez, G.; Elnitsky, M.A.; Lee, R.E., Jr.; Denlinger, D.L. Metabolomics reveals unique and shared metabolic changes in response to heat shock, freezing and desiccation in the Antarctic midge, Belgica Antarctica. J. Insect Physiol. 2008, 54, 645–655.
- Turner, J.; Lu, H.; King, J.; Marshall, G.J.; Phillips, T.; Bannister, D.; Colwell, S. Extreme temperatures in the Antarctic. J. Clim. 2021, 34, 2653–2668.
- Vanstreels, R.E.T.; Palma, R.L.; Mironov, S.V. Arthropod parasites of Antarctic and sub-Antarctic birds and pinnipeds: A review of host-parasite associations. Int. J. Parasitol. Parasites Wildl. 2020, 12, 275–290.
- Hodkinson, I.D.; Coulson, S.J.; Webb, N.R. Community assembly along proglacial chronosequences in the high Arctic: Vegetation and soil development in north-west Svalbard. J. Ecol. 2003, 91, 651–663.
- Giribet, G.; Edgecombe, G.D. The phylogeny and evolutionary history of arthropods. Curr. Biol. 2019, 29, R592–R602.
- Maurice, H.; Philippe, V. Terrestrial macro-arthropods of the sub-Antarctic islands of Possession (Crozet Archipelago) and Kerguelen: Inventory of native and non-native species. Zoosystema 2021, 43, 549–561.
- Sinclair, B.J.; Terblanche, J.S.; Scott, M.B.; Blatch, G.L.; Jaco Klok, C.; Chown, S.L. Environmental physiology of three species of Collembola at Cape Hallett, North Victoria Land, Antarctica. J. Insect Physiol. 2006, 52, 29–50.
- Høye, T.T.; Forchhammer, M.C. Phenology of high-Arctic arthropods: Effects of climate on spatial, seasonal, and inter-annual variation. In Advances in Ecological Research; Academic Press: Cambridge, MA, USA, 2008; Volume 40, pp. 299–324.
- Sinclair, B.J.; Coello Alvarado, L.E.; Ferguson, L.V. An invitation to measure insect cold tolerance: Methods, approaches, and workflow. J. Therm. Biol. 2015, 53, 180–197.
- Rozsypal, J.; Košťál, V. Supercooling and freezing as eco-physiological alternatives rather than mutually exclusive strategies: A case study in Pyrrhocoris apterus. J. Insect Physiol. 2018, 111, 53–62.
- Duman, J.G.; Bennett, V.; Sformo, T.; Hochstrasser, R.; Barnes, B.M. Antifreeze proteins in Alaskan insects and spiders. J. Insect Physiol. 2004, 50, 259–266.
- Worland, M.R. Factors that influence freezing in the sub-Antarctic springtail Tullbergia antarctica. J. Insect Physiol. 2005, 51, 881–894.
- Zettel, J. The significance of temperature and barometric pressure changes for the snow surface activity of Isotoma hiemalis (Collembola). Experientia 1984, 40, 1369–1372.
- Sinclair, B.J.; Sjursen, H. Cold tolerance of the Antarctic springtail Gomphiocephalus hodgsoni (Collembola, Hypogastruridae). Antarct. Sci. 2001, 13, 271–279.
- Rinehart, J.P.; Li, A.; Yocum, G.D.; Robich, R.M.; Hayward, S.A.L.; Denlinger, D.L. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc. Natl. Acad. Sci. USA 2007, 104, 11130–11137.
- Storey, K.B.; Storey, J.M. Insect cold hardiness: Metabolic, gene, and protein adaptation. Can. J. Zool. 2012, 90, 456–475.
- Walters, K.R., Jr.; Sformo, T.; Barnes, B.M.; Duman, J.G. Freeze tolerance in an arctic Alaska stonefly. J. Exp. Biol. 2009, 212, 305–312.
- Clark, M.S.; Thorne, M.A.S.; Purać, J.; Burns, G.; Hillyard, G.; Popović, Ž.D.; Grubor-Lajšić, G.; Worland, M.R. Surviving the cold: Molecular analyses of insect cryoprotective dehydration in the Arctic springtail Megaphorura arctica (Tullberg). BMC Genom. 2009, 10, 328.
- Sørensen, J.G.; Holmstrup, M. Cryoprotective dehydration is widespread in Arctic springtails. J. Insect Physiol. 2011, 57, 1147–1153.
- Sformo, T.; Walters, K.; Jeannet, K.; Wowk, B.; Fahy, G.M.; Barnes, B.M.; Duman, J.G. Deep supercooling, vitrification and limited survival to –100°C in the Alaskan beetle Cucujus clavipes puniceus (Coleoptera: Cucujidae) larvae. J. Exp. Biol. 2010, 213, 502–509.
- Elnitsky, M.A.; Hayward, S.A.; Rinehart, J.P.; Denlinger, D.L.; Lee, R.E., Jr. Cryoprotective dehydration and the resistance to inoculative freezing in the Antarctic midge, Belgica antarctica. J. Exp. Biol. 2008, 211, 524–530.
- Teets, N.M.; Gantz, J.D.; Kawarasaki, Y. Rapid cold hardening: Ecological relevance, physiological mechanisms and new perspectives. J. Exp. Biol. 2020, 223, jeb203448.
- Teets, N.M.; Denlinger, D.L. Physiological mechanisms of seasonal and rapid cold-hardening in insects. Physiol. Entomol. 2013, 38, 105–116.
- Worland, M.R.; Convey, P. Rapid cold hardening in Antarctic microarthropods. Funct. Ecol. 2001, 15, 515–524.
- Hawes, T.C.; Couldridge, C.E.; Bale, J.S.; Worland, M.R.; Convey, P. Habitat temperature and the temporal scaling of cold hardening in the high Arctic collembolan, Hypogastrura tullbergi (Schäffer). Ecol. Entomol. 2006, 31, 450–459.
- Clarke, A.; Fraser, K.P.P. Why does metabolism scale with temperature? Funct. Ecol. 2004, 18, 243–251.
- Schulte, P.M. The effects of temperature on aerobic metabolism: Towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Exp. Biol. 2015, 218, 1856–1866.
- Williams, C.M.; Szejner-Sigal, A.; Morgan, T.J.; Edison, A.S.; Allison, D.B.; Hahn, D.A. Adaptation to low temperature exposure increases metabolic rates independently of growth rates. Integr. Comp. Biol. 2016, 56, 62–72.
- Terblanche, J.S.; Clusella-Trullas, S.; Deere, J.A.; Van Vuuren, B.J.; Chown, S.L. Directional evolution of the slope of the metabolic rate–temperature relationship is correlated with climate. Physiol. Biochem. Zool. 2009, 82, 495–503.
- Addo-Bediako, A.; Chown, S.L.; Gaston, K.J. Metabolic cold adaptation in insects: A large-scale perspective. Funct. Ecol. 2002, 16, 332–338.
- Ayres, M.P.; Scriber, J.M. Local adaptation to regional climates in Papilio canadensis (Lepidoptera: Papilionidae). Ecol. Monogr. 1994, 64, 465–482.
- Lardies, M.A.; Bacigalupe, L.D.; Bozinovic, F. Testing the metabolic cold adaptation hypothesis: An intraspecific latitudinal comparison in the common woodlouse. Evol. Ecol. Res. 2004, 6, 567–578.
- Nespolo, R.F.; Lardies, M.A.; Bozinovic, F. Intrapopulational variation in the standard metabolic rate of insects: Repeatability, thermal dependence and sensitivity (Q10) of oxygen consumption in a cricket. J. Exp. Biol. 2003, 206, 4309–4315.
- MacMillan, H.A.; Sinclair, B.J. Mechanisms underlying insect chill-coma. J. Insect Physiol. 2011, 57, 12–20.
- David, J.R.; Gibert, P.; Moreteau, B.; Gilchrist, G.W.; Huey, R.B. The fly that came in from the cold: Geographic variation of recovery time from low-temperature exposure in Drosophila subobscura. Funct. Ecol. 2003, 17, 425–430.
- Marden, J.H. Nature’s inordinate fondness for metabolic enzymes: Why metabolic enzyme loci are so frequently targets of selection. Mol. Ecol. 2013, 22, 5743–5764.
- Noer, N.K.; Sørensen, M.H.; Colinet, H.; Renault, D.; Bahrndorff, S.; Kristensen, T.N. Adjustments in thermal tolerance and the metabolome to daily environmental changes-a field study of the arctic seed bug Nysius groenlandicus. Front. Physiol. 2022, 13, 818485.
- Horne, C.R.; Hirst, A.G.; Atkinson, D. Seasonal body size reductions with warming covary with major body size gradients in arthropod species. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170238.
- Verberk, W.C.E.P.; Atkinson, D.; Hoefnagel, K.N.; Hirst, A.G.; Horne, C.R.; Siepel, H. Shrinking body sizes in response to warming: Explanations for the temperature–size rule with special emphasis on the role of oxygen. Biol. Rev. 2021, 96, 247–268.
- Klok, C.J.; Harrison, J.F. The temperature size rule in arthropods: Independent of macro-environmental variables but size dependent. Integr. Comp. Biol. 2013, 53, 557–570.
- Bowden, J.J.; Eskildsen, A.; Hansen, R.R.; Olsen, K.; Kurle, C.M.; Høye, T.T. High-Arctic butterflies become smaller with rising temperatures. Biol. Lett. 2015, 11, 20150574.
- Aguilar-Alberola, J.A.; Mesquita-Joanes, F. Breaking the temperature-size rule: Thermal effects on growth, development and fecundity of a crustacean from temporary waters. J. Therm. Biol. 2014, 42, 15–24.
- Scriven, J.J.; Whitehorn, P.R.; Goulson, D.; Tinsley, M.C. Bergmann’s body size rule operates in facultatively endothermic insects: Evidence from a complex of cryptic bumblebee species. PLoS One 2016, 11, e0163307.
- Shelomi, M. Where are we now? Bergmann’s rule sensu lato in insects. Am. Nat. 2012, 180, 511–519.
- Danks, H.V. Seasonal adaptations in Arctic insects. Integr. Comp. Biol. 2004, 44, 85–94.
More
