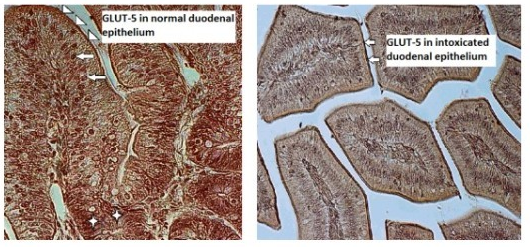
Although patterns of glucose transporter expression and notes about diseases leading to adaptive changes in intestinal fructose transport have been well-characterized, the connection between infection and fructose transportation has been lightly investigated. Up to now only few studies on GLUT-5 expression and function under pathological conditions in bird intestines have been carried out. TIn the aim of our current research was to study we immunolocalized GLUT-5 in one-week-old chicken (Gallus gallus domesticus) duodenal epithelium in norm and during T-2 mycotoxicosis using polyclonal primary antibody Rabbit anti-GLUT-5 (Abcam, UK). OurThe study revealed the strong expression of GLUT-5 in the apical parts of the duodenal epithelial cells in the control group chickens and weak staining for GLUT-5 in the intestinal epithelium in the T-2 mycotoxicosis group indicating to the decreased expression of GLUT-5 in the duodenal epithelium during T-2 mycotoxicosis.
- glucose transporters
- GLUT-5
- T-2 mycotoxin
- T-2 mycotoxicosis
- duodenal epithelium
- Gallus gallus domesticus
Introduction
Glucose transporters and GLUT-5
Glucose, being a main energy source [1], and glucose transporters being present in all phyla, the proteins belonging to the GLUT or SLC2A family are found in most mammalian cells. The main role in transmembrane monosaccharide transport belongs to the glucose transporter (GLUT) proteins, which influence blood sugar regulation [2][3]. Glucose reaches into the target cells, passing through the intestinal epithelium. Glucose is absorbed by two structurally and functionally different groups, exhibiting different substrate specificities, kinetic properties and tissue expression profiles: active—absorption mediated by sodium-dependent glucose transporters (SGLTs); diffusive—glucose transporter facilitators (GLUTs) [4][5]. The GLUT family can be divided into three subclasses on the basis of sequence similarities: Class I—glucose transporters; Class II—formed by fructose transporters; Class III—belong structurally atypical transporters [6].
The knowledge of glucose transporters in avians is fragmented. Glucose transporters in avians
It is known that diets of many birds change with seasons, causing the carbohydrate levels to vary. Comparing the dietary regulations of intestinal glucose transport with other species (fishes, amphibians, mammals) the transport in birds does not change, which may be caused by the predominance of passive glucose transport [7]. It is also known that birds maintain higher plasma glucose concentrations than other vertebrates of similar body mass and are mostly insensitive to the regulation of plasma glucose concentrations by insulin [4]. Glucose is absorbed by the avian gastrointestinal tract by sodium–glucose transporters and glucose transport proteins. In the small intestine, four of the most abundant transporters are found: GLUT-1, GLUT-2, GLUT-5 and SGLUT1 [8], of which GLUT-5 is a high affinity fructose transporter. The main site for GLUT-5 absorption is the epithelium of jejunum and in the last part of ileum [9][10], as the epithelial cells absorb hexoses from the intestinal lumen and export sugars into blood. In the beginning, absorption depends on active transport in the apical brush border membrane, after which the hexoses move out of the enterocytes by passive transport [11].
Glucose transporter-5 (GLUT-5)
Glucose transporter-5 (GLUT-5),is the main apical fructose transporter and the transporter which is specific only for fructose, allows fructose, the sweetest of all natural sugars, to be transported from the intestinal lumen into the enterocyte by facilitated diffusion, due to fructose’s high concentration in the intestinal lumen [12]. According to the literature, there are studies which support the direct relationship between increases in the consumption of fructose and the increase in the diabetes type-2 [13]. The previous studies have shown that the small intestine regulates fructose absorption from dietary sources and expresses the greatest amount of GLUT-5 in mammals [14].
Although it is known that infectious diseases cause changes in intestinal absorptive function, the connection of infection and fructose transport has been slightly studied. Up to now, only few studies on intestinal GLUT-5 expression and function during diseases have been carried out [15][16].
GLUT-2 mycotoxin and5 in T-2 mycotoxicosis
T-2 mycotoxin, the basic type A trichothecene mycotoxin, has been regarded to be the most toxic trichothecene. In dynamic cell proliferation tissues, such as the gastrointestinal tissues, T-2 mycotoxin poses different toxic effects [17][18]. It was shown that, in poultry, the T-2 toxin elicits genetic, cellular toxic and immunomodulatory effects, influencing the cells of the digestive, nervous and integumentary system, as well as on the impairment of poultry performance [19]. Symptoms of T-2 mycotoxicosis in chicken manifest in growth retardation, lower feed intake, reduced egg production with thinner eggshells, impaired egg hatch, leucopenia and the cyanosis of the comb. Besides, T-2 mycotoxin is among to the most essential trichothecene mycotoxins, which occurs in several agricultural products [20], causing severe diseases and even death among humans and animals [21]. Animals are exposed through food to T-2 mycotoxins, whose initial interaction is with the gut epithelium.
As animals are exposed through food to T-2 mycotoxins, one of the most deadly toxins of the trichothecene group and, due to the absence of data about the effect of mycotoxicosis on GLUT-5 in the intestines of birds during their first post-hatching week, theGLUT-5 aim of our current research was to was immunolocalize GLUT-5d in our study in the seven-day old chicken duodenal epithelium in norm and during T-2 mycotoxicosis.
Discussion
In the gastrointestinal system, the glucose transporter expression is the greatest in the small intestine, where the absorption for monosaccharides depends on the sodium-dependent glucose transporter SGLUT1 and the facilitated-diffusion glucose transporters located in the intestinal epithelium [22][23]. Identical expressions of GLUT2 and GLUT5 mRNA have been noticed from the proximal to middle parts of the small intestine [23][24] where GLUT-5 is located on the apical membrane of epithelial cells. While galactose and glucose transport is mediated by SGLT1 [7][25][26], GLUT-5 only mediates the uptake of fructose [27][28][29], whose activity changes during pathological conditions. Generally, it was found that the activity and expression of GLUT-5 was reduced during inflammation and sepsis in rabbits [30][31]. According to those authors lipopolysaccharide and tumor necrosis factor-α, as the main causes of sepsis, provoked decreased fructose absorption in the jejunum. In humans it has been noted that the infection caused by Helicobacter pylori also reduces the expression of intestinal GLUT-5 [16]. Decreased expression of GLUT-5 in the duodenal epithelial cells in the T-2 mycotoxicosis group found in our study points towards reduced fructose transportation in the diseased gut epithelium. The gastrointestinal organs are among the first organs coming into contact with mycotoxins of dietary origin. T-2 mycotoxin is a naturally occurring mold byproduct of Fusarium spp. fungus, toxic both to humans and animals. The ingestion of T-2 mycotoxin may occur because of the intake of moldy grains—barley, maize, rice, wheat, etc. The T-2 mycotoxin is specific because the systemic toxicity can result from different route of exposure—respiratory, oral and dermal [32]. The T-2 toxin can be absorbed through human skin, unlike most biological toxins [33]. Besides skin, it causes symptoms related to respiratory and gastrointestinal organs. The fact that it is delivered by water, droplets, aerosols from various dispersal systems and food also makes it a potential biological weapon [34]. In vivo and in vitro T-2 mycotoxin can inhibit DNA and RNA, as well as inhibit protein synthesis 35 [35]. These effects led to apoptosis in various tissues, including immune- and gastrointestinal systems [36]. In immune systems, it inhibits erythropoiesis in the bone marrow and spleen by disturbing the antibody production [37]. According to earlier research, T-2 mycotoxin is able to inhibit IL-2 and IL-5 production by T cells. It has been reported that low concentrations of T-2 mycotoxin ingestion changes Toll-like receptor activation, interfering with the initiation of the inflammatory immune
responses against viruses and bacteria. Thus, the mycotoxins may increase the receptivity of animals and humans to infectious diseases [38][39]. Mycotoxins elicit similar toxic effects among humans and animals. In bird intestines, various methods have been used to study the effects of toxins [40][41]. Some data revealed the immuno- and cytotoxic effect of ochratoxin A on intestinal epithelium and MALT-system (mucosa-associated lymphoid tissue), modifying the intestinal barrier and thus increasing receptiveness to different associated diseases [35]. A decreased glucose uptake has been registered after the oral administration of the T-2 toxin [42].
In our recent study decreased expression of GLUT-5 immunolocalized in the duodenal epithelial cells in one-week-old T-2 toxicated broilers, compared to the control group points towards reduced fructose transportation in the diseased gut epithelium after only three days of T-2 mycotoxin administration.
References
- Stevens, L. Avian Biochemistry and Molecular Biology; Cambridge University Press: Cambridge, UK, 1996; pp. 29–45.
- Welch, K.C., Jr.; Allalou, A.; Sehgal, P.; Cheng, J.; Ashok, A. Glucose transporter expression in an avian nectarivore: The ruby-throated hummingbird (Archilochus colubris). PLoS ONE 2013, 8, e77003.
- Takata, K. Glucose transporters in the transepithelial transport of glucose. J. Electron. Microsc. 1996, 45, 275–284.
- Braun, E.J.; Sweazea, K.L. Glucose regulation in birds. Comp. Biochem. 2008, 151, 1–9.
- Wood, I.S.; Trayhurn, P. Glucose transporters (GLUT and SGLT): Expanded families of sugar transport proteins. Br. J. Nutr. 2003, 89, 3–9.
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Aspects Med. 2013, 34, 121–138.
- Ferraris, R.P. Dietary and developmental regulation of intestinal sugar transport. Biochem. J. 2001, 360, 265–
- Yoshikawa, T.; Inoue, R.; Matsumoto, M.; Yajima, T.; Ushida, K.; Iwanaga, T. Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. Histochem. Cell Biol. 2011, 135, 183–194.
- Mueckler, M. Facilitative glucose transporters. Eur. J. Biochem. 1994, 219, 713–725.
- Augustin, R. The protein family of glucose transport facilitators: it’s not only about glucose after all. IUBMB Life 2010, 62, 315–333.
- Thorens, B. Facilitated glucose transporters in epithelial cells. Annu. Rev. Physiol. 1993, 55, 591–608.
- Kellett, G.L.; Brot-Laroche, E. Apical GLUT2. A major pathway of intestinal sugar absorption. Diabetes 2005, 54, 3056–3062.
- Ang, B.R.G.; Yu, G.F. The Role of Fructose in Type 2 Diabetes and other Metabolic Diseases. J. Nutr. Food Sci. 2018, 8, 659.
- Gilbert, E.R.; Li, H.; Emmerson, D.A.; Webb, K.E., Jr.; Wong, E.A. Developmental regulation of nutrient transporter and enzyme mRNA abundance in the small intestine of broilers. Poult. Sci. 2007, 86, 1739–1753.
- Wasserman, D.; Hoekstra, J.H.; Tolia, V.; Taylor, C.J.; Kirschner, B.S.; Takeda, J.; Bell, G.I.; Taub, R.; Rand, E.B. Molecular analysis of the fructose transporter gene (GLUT5) in isolated fructose malabsorption. J. Clin. Investig. 1996, 98, 2398–2402.
- Lertanekawattana, S.; Wichatrong, T.; Chaisiri, K.; Uchikawa, R.; Arizono, N. Expression of cytokines and monosaccharide transporters in the duodenal mucosa of patients with gastrointestinal symptoms in rural Thailand. Southeast Asian J. Trop. Med. Public Health 2005, 36, 923–930.
- Kumagai, S.; Shimizu, T. Effects of Fusarenon-X and T-2 toxin on intestinal absorption of monosaccharide in rats. Arch. Toxicol. 1998, 61, 489–495.
- Zhang, Y.; Han, Y.; Zhu, C.C.; Tang, F.; Cui, X.-S.; Kim, N.; Sun, S.-C. Exposure to HT-2 toxin causes oxidative stress induced apoptosis/autophagy in porcine oocytes. Sci. Rep. 2016, 6, 33904.
- Sokolović, M.; Garaj-Vrhovac, V.; Šimpraga, B. T-2 toxin incidence and toxicity in poultry. Arh. Hig. Rada Toksikol. 2008, 59, 43–52.
- Yuan, G.; Wang, Y.; Yuan, X.; Zhang, T.; Zhao, Y.; Huang, L.; Peng, S. T-2 toxin induces developmental toxicity and apoptosis in zebrafish embryos. J. Environ. Sci. 2014, 26, 917–925.
- Kachuei, R.; Rezaie, S.; Hossein Yadegari, M.; Safaie, N.; Allameh, A.-A.; Aref-Poor, M.-A.; Imani Fooladi, A.-A.; Riazipour, M.; Mohammad Abadi, H.M. Determination of T-2 Mycotoxin in Fusarium strains by HPLC with fluorescence detector. J. Appl. Biotechnol. Rep. 2014, 1, 38–43.
- Merigo, F.; Brandolese, A.; Facchin, S.; Missaggia, S.; Bernardi, P.; Boschi, F.; D’Inca, R.; Savarino, E.V.; Sbarbati, A.; Sturniolo, G.C. Glucose transporter expression in the human colon. World J. Gastroenterol. 2018, 24, 775–793.
- Hussar, P.; Kärner, M.; Järveots, T.; Pendovski, L.; Dūrītis , I.; Popovska-Percinic, F. Comparative study of glucose transporters GLUT-2 and GLUT-5 in ostriches gastrointestinal tract. Maced. Vet. Rev. 2016, 39, 225−231.
- Hussar, P.; Kärner, M.; Dūrītis, I.; Plivča, A.; Pendovski, L.; Järveots, T.; Popovska-Percinic, F. Temporospatial study of hexose transporters and mucin in the epithelial cells of chicken (Gallus gallus domesticus) small intestine. Pol. J. Vet. Sci. 2017, 20, 627–633.
- Dong, R.; Srai, S.K.; Debnam, E.; Smith, M. Transcriptional andtranslational control over sodium-glucoselinked transporter(SGLT1) gene expression in adult rat small intestine. FEBS Lett. 1997, 406, 79–82.
- Kojima, T.; Nishimura, M.; Yajima, T.; Kuwata, T.; Suzuki, Y.; Goda, T.; Takase, S.; Harada, E. Developmental changes in theregional Na+/glucose transporter mRNA along the smallintestine of suckling rats. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998, 122, 89–95.
- Burant, C.F.; Saxena, M. Rapid reversible substrate regulation of fructose transporter expression in rat small intestine and kidney. Am. J. Physiol. Gastrointest. Liver Physiol. 1994, 267, G71–G79.
- Burant, C.F.; Takeda, J.; Brot-Laroche, E.; Bell, G.I.; Davidson, N.O. Fructose transporter in human spermatozoa and small intestine is GLUT5. J. Biol. Chem. 1992, 267, 14523–14526.
- Douard, V.; Ferraris, R.P. Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E227–E237.
- Garcia-Herrera. J.; Abad, B.; Rodriguez-Yoldi, M.J. Effect of lipopolysaccharide on d-fructose transport across rabbit jejunum. Inflamm. Res. 2003, 52, 177–184.
- Garcia-Herrera, J.; Navarro, M.A.; Marca, M.C.; de la Osada, J.; Rodriguez-Yoldi, M.J. The effect of tumor necrosis factor-alpha on d-fructose intestinal transport in rabbits. Cytokine 2004, 25, 21–30.
- Afsah-Hejri, L.; Jinap, S.; Hajeb, P.; Radu, S.; Shakibazadeh, S.H. A review on mycotoxins in food and feed: Malaysia case study. Compr. Rev. Food Sci. Food Saf. 2013, 12, 629–651.
- Boonen, J.; Malysheva, S.V.; Taevernier, L.; Di Mavungu, J.D.; De Saeger, S.; De Spiegeleer, B. Human skin penetration of selected model mycotoxins. Toxicology 2012, 301, 21–32.
- Caldwell, R.D. Yellow rain’ or natural toxins? Nature 1983, 301, 651.
- Adegoke, G.O.; Letuma, P. Strategies for the Prevention and Reduction of Mycotoxins in Developing Countries, Mycotoxin and Food Safety in Developing Countries. In Mycotoxin and Food Safety in Developing Countries; IntechOpen: Rijeka, Croatia, 2013; pp. 123–136.
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237.
- Torp, M.; Langseth, W. Production of T-2 toxin by a Fusarium resembling Fusarium poae. Mycopathologia 1999, 147, 89–96.
- Obremski, K.; Podlasz, P.; Żmigrodzka, M.; Winnicka, A.; Woźny, M.; Brzuzan, P.; Jakimiuk, E.; Wojtacha, P.; Gajęcka, M.; Zielonka, L.; et al. The effect of T-2 toxin on percentages of CD4+, CD8+, CD4+CD8+ and CD21+ lymphocytes, and mRNA expression levels of selected cytokines in porcine ileal Peyer’s patches. Pol. J. Vet. Sci. 2013, 16, 341–349.
- Seeboth, R.; Solinhac, I.P.; Oswald, L.; Guzylack, P. The fungal T-2 toxin alters the activation of primary macrophages induced by TLR-agonists resulting in a decrease of the inflammatory response in the pig. Vet. Res. 2012, 43, 35.
- Sweazea, K.L.; Braun, E.J. Glucose transporter expression in English sparrows (Passer domesticus). Comparative Biochemistry and physiology. Part. B Biochem. Mol. Biol. 2006, 144, 263–270.
- Wang, F.; Zuo, Z.; Chen, K.; Gao, C.; Yang, Z.; Zhao, S.; Li, J.; Song, H.; Peng, X.; Fang, J.; et al. Histopathological Injuries, Ultrastructural Changes, and Depressed TLR Expression in the Small Intestine of Broiler Chickens with Aflatoxin B1. Toxins 2018, 10, 131.
- Phletus, P.W. Effects of T-2 mycotoxin on gastrointestinal tissues: A Review of in vivo and in vitro models.Arch. Environ. Contam. Toxicol. 1989, 18, 374–387.