[1]1. Nociceptors and Peripheral Nerves
A noxious stimulus might cause tissue damage. Physiologically, it results in pain perception by the conscious subject. The condition causing pain often relates to tissue injury (wounds, fractures…), inflammation, traction, etc. At the very onset of perception, the complexity of the pain phenomenon begins.
In the 1930s, Adrian [
6] was among the first to analyze the activity of sensory receptors. Impulses through Aδ and unmyelinated C fibers related to painful stimuli. The primary receptor involved in pain perception is a free nerve ending, which transmits information more slowly. However, there are also specific mechanical-, thermal-, or polymodal-sensory nociceptors (which respond to mechanical, thermal, or chemical stimuli) in the skin, viscera, and muscle [
7].
The activation of Aδ fibers is associated with rapid, well-defined pain, and C fibers are responsible for secondary pain sensations, which are slow, diffuse, and of longer duration. In general, large fibers have a lower stimulation threshold, both natural and electrical. A stimulus intense enough to activate thin unmyelinated fibers surpasses that needed to stimulate the large fibers, conveying a different pain sensation. Therefore, an elective response to a noxious stimulus in the skin may not appear, transmitting uncertain information about painful stimulation. Several theories have been put forward about this problem of sensory transmission [
8]: (1) Theory of the specificity of sensation, by Mac von Frey [
9], who stated that each type of receptor transmits information about its specific stimulus; (2) Goldscheider’s stimulus summation theory (1894), in which he proposed that pain is produced by the summation of impulses within the CNS, after applying mechanical or thermal stimuli to the skin; and (3) Nafe’s theory of impulse behavior (1934), i.e., sensation is not based on specific receptors, but on the way in which a given number of impulses are transferred in space (number and type of fibers) and time (frequency). Melzack and Wall [
10] incorporated and modified this theory, maintaining an eclectic position, adding the possibility of the existence of stimulus specificity and the absence of stimuli.
The majority opinion was reviewed by Kerr and Wilson [
11]. Faced with the alternative of the specificity of pain information or dependence on stimulus behavior, the majority acknowledge a specific system in which nociceptors, with their corresponding afferent fibers, activate different types of neurons in the CNS. Some nociceptors are high-threshold units (responding only to noxious stimuli). But for others, noxious and non-noxious stimuli converge, veiling nociceptor specificity. In this sense, C fibers associated with pain and pleasure have been differentiated: low threshold, tactile, pleasure [
12] and high threshold, tactile, pain [
13].
2. Spinal Ganglion-Dorsal Root
At the level of the spinal ganglion, there are two types of cells. The larger ones seem to give rise to myelinated axons, and the small ones give rise to unmyelinated axons. The latter are nociceptive and can be subdivided into two types [
14], depending on whether they contain substance P or somatostatin as a neurotransmitter. Randić and Miletić [
15] suggest that substance P may play a role as an excitatory transmitter at the level of the first synapse, and that somatostatin may have an inhibitory modulatory effect.
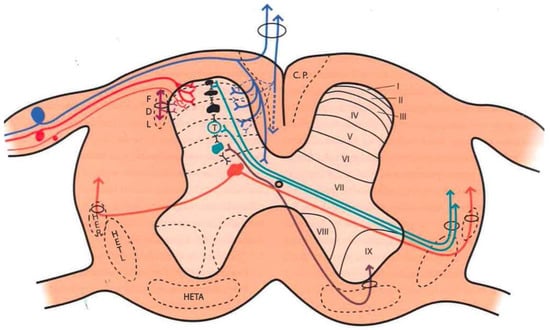
Large fibers converge in a medial tract and penetrate the posterior spinal cords, sending collaterals in small proportion to the dorsomedial area of the dorsal horn, at the level of the substantia gelatinosa of Rolando. The rest end up at the level of Rexed’s laminae IV and V and in the intermediate gray matter (Figure 1).
Figure 1. Distribution of afferent pathways in the spinal cord (see text). (Taken from [
16]). C.P. = P.C. (Posterior cord). HER = SRT (Spinoreticular tract). HETL = LSTT (Lateral spinothalamic tract). HETA = VSTT (Ventral spinothalamic tract). FDL = DLF (Dorsolateral fasciculus, Lissauer’s tract). I-IX = Laminae of spinal cord gray matter.
Aδ fibers and C fibers converge into two bundles: a larger one on the ventrolateral portion of the posterior root and a second, smaller bundle on the dorsomedial surface. Both types of fibers reach the Lissauer tract and are distributed mainly at the level of laminae I, II, and V.
3. Dorsal Horn of the Spinal Cord
The spinal cord gray matter was initially differentiated by Rexed [
17], depending on the cytoarchitecture, into ten laminae, of which the first seven are involved in the pain phenomenon (
Figure 1 and
Figure 2):
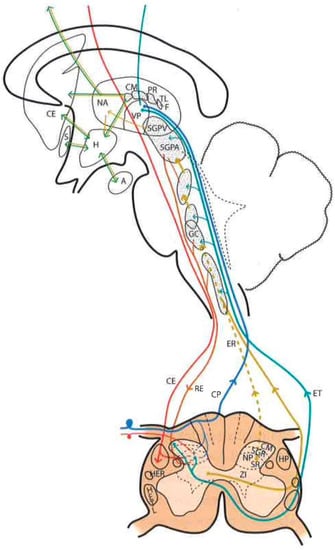
Figure 2. Diagram of the distribution of the main sensory pathways involved in pain perception and control (see text). (Taken from [
16]). SGPV = PVG (Periventricular Gray Matter). SGPA = PAG (Periaqueductal Gray Matter). H = H (Hypothalamus). CE = CS (Corticospinal), RE = RS (Reticulospinal), ER = SR (Spinoreticular) and HETL = LSTT (Lateral spinothalamic) Tracts. NP = PN (Pulvinar nucleus). GM = MG (Medial geniculate nucleus).
-
Lamina I (marginal layer of Waldeyer) consists of large neurons whose afferents are mostly formed by Aδ fibers and, to a lesser extent, unmyelinated C fibers [
18]. They project to the ventral posterior (VP) and intralaminar thalamic nuclei as well as to the periaqueductal gray matter (PAG) [
19,
20]. These are type I neurons, as described by Iggo [
21], responding to painful or almost noxious stimuli.
-
Laminae II and III (substantia gelatinosa of Rolando) contain small-sized neurons receiving most afferents through C fibers, and the rest through collaterals of Aβ fibers of the posterior spinal cord [
18]. Their extensions form part of Lissauer’s dorsolateral fasciculus along three or more segments. Their function is still obscure, acting as interneurons and maintaining an inhibitory or excitatory influence on the cells of laminae I, IV, and V [
22].
-
The cells of laminae IV, V, and VI (nucleus proprius, neck, or reticular substance and base, respectively, of the dorsal horn) are Iggo type II nociceptive neurons, which respond to low-threshold peripheral mechanical stimuli, but increase their firing frequency when the stimulus becomes noxious [
23]. For Mayer and Price [
24], the activation of these type II neurons can lead to experiencing pain. From these laminae originates the spinothalamic tract, which projects to the sensory ventral posteromedial and posterolateral (VPM and VPL) thalamic nuclei, as well as PAG.
-
Lamina VII (intermediate zone) gives rise to the spinoreticular tract, next to the spinothalamic tract, and its neurons respond to stimuli that trigger the highest-threshold receptors [
25].
4. Pain Information Pathways
At least three types of ascending pathways transmit pain information to the brain (Figure 1 and Figure 2):
4.1. Neospinothalamic Tract or Lateral Spinothalamic Tract
Phylogenetic modifications are needed to improve the direct transmission of pain impulses to conscious levels. Such modifications seem to originate at the level of laminae I, IV, VI, and VII [
26]; the fibers cross the midline at the level of the anterior white commissure and ascend through the anterolateral quadrant of the spinal cord. They project, after forming part of the lemniscus medialis, to the level of the sensory thalamic nuclei (VPL, the area of the body; VPM, the area of the face) and from here to the postrolandic sensory cortex, transmitting information about the discriminative aspects of the pain experience.
4.2. Paleospinothalamic Tract
It originates in the same type I and II neurons as the previous pathway, but the difference is that they give numerous collaterals to brainstem reticular formation. A multisynaptic pathway is formed that ascends to areas related to behavior (hypothalamus, intralaminar, parafascicular, central medial (CM) thalamic nuclei, and the limbic circuit). It projects bilaterally and seems to be involved in the motivational and affective qualities of pain [
7].
4.3. Archispinothalamic Tract
Much less differentiated, it appears to be an ascending multisynaptic pathway at the level of the medial medullary reticular substance to diencephalic and cortical zones similar to the paleospinothalamic tract, including the limbic system [
27].
In addition, we must consider a descending pain system, which seems to be related to pain inhibition. It was discovered after observing that the PAG stimulation in rats produced analgesia [
28], a phenomenon called SPA (stimulation-produced analgesia) [
29] that is also seen in humans [
30]. This descending inhibitory system, acting on the dorsal horns, is conveyed by the posterior spinal cords and reticulospinal and corticospinal pathways. However, as we will see later, there are inhibitory circuits at the level of each station or relay of the pain pathway (dorsal horn, brainstem, thalamus, etc.).
5. Brainstem Reticular Formation
It is difficult to obtain a strict scientific definition of the criteria necessary to designate a certain area of the CNS as “reticular”. By exclusion, and unlike well-delimited nuclei and fiber tracts, there are areas where gray and white matter intermingle, fibers form bundles in all directions, and neurons are diffusely distributed, forming poorly defined clusters. Current knowledge of neuronal morphology and connections may not allow us to appreciate a certain organization that actually exists. Most authors include areas located deep at the level of the medulla oblongata, pons, and midbrain as the reticular formation (RF). There is less agreement with regard to including central regions of the spinal cord gray matter, nonspecific thalamic nuclei, and certain hypothalamic nuclei (Figure 2).
The RF is considered to be the oldest phylogenetically, representing the “free neural network” on which more circumscribed and highly organized parts of the CNS have subsequently appeared. However, even the most primitive CNSs have diffuse or highly organized zones. Therefore, it is preferable to look at the RF as the evolution of these more diffusely organized zones or elements, with the following general characteristics: (a) they are deeply located groups of neurons and fibers with a diffuse structural organization; (b) connecting pathways are anatomically difficult or impossible to define, or physiologically complex and often polysynaptic; (c) components of both ascending and descending systems can be recognized; (d) both systems contain crossed and uncrossed elements, eliciting ipsi- and/or contralateral responses after stimulation; and (e) they intervene in somatic and visceral functions
In this complex system, there are massive opportunities for the convergence or divergence of information. In addition, neurons are either excitatory or inhibitory, or cholinergic or aminergic, varying their proportion depending on the region. Importantly, one of the many functions of the RF is short- and long-term homeostasis, with its descending pathways to lower autonomic centers and its ascending pathways carrying visceral and somatic information to the hypothalamus and limbic system. In this sense, we can find centers related to cardiovascular, respiratory, and gastrointestinal control mechanisms as well as to the ascending, bilateral, nociceptive pathways of “slow pain”. Concerning this type of pain, certain regions have been identified (Figure 2):
-
Gigantocellular reticular nucleus and lateral reticular region, at the level of the medulla oblongata and pons—they receive projections from the spinothalamic tract as well as from the deeper areas of spinal cord gray matter [
31].
-
Nuclei raphe magnus and PAG, at the level of the midbrain [
19,
20]—stimulation of the latter in humans causes somatic and visceral sensations accompanied by intense emotional reactions.
-
Ventrolateral PAG and periventricular gray matter (PVG)—lateral to the third ventricle, in the region of the posterior commissure [
28], are the areas whose stimulation produces the most effective analgesia with minimal side effects. This SPA [
26] may last for several hours after stimulation, suggesting the activation of a multisynaptic system with a mechanism similar to opioid analgesia [
32]. Both areas are, indeed, rich in opioid receptors, and their stimulation causes the release of endorphins in CSF. There is, on the other hand, cross-tolerance between SPA and opioid analgesia, just as SPA can be reversed by naloxone [
33].
6. Thalamus
The thalamic structures that directly receive projections of the spinothalamic pain pathway correspond to three groups of nuclei [
34] (
Figure 2 and
Figure 3):
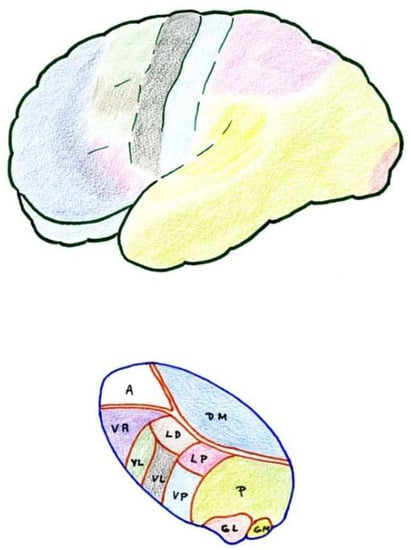
Figure 3. Anatomical–functional correlation between the thalamus and cerebral cortex (see text). LD (Lateral dorsal nucleus). LP (Lateral posterior nucleus). VL (Ventral lateral nucleus). VA (Ventral anterior nucleus). VL = VPL (Ventral posterolateral). VP = VPM (Ventral posteromedial). A = AN (Anterior nuclear group). DM = MD (Medial dorsal nucleus). P (Pulvinar). GL = LG (Lateral geniculate nucleus). GM = MG (Medial geniculate nucleus).
6.1. Ventrobasal Complex
The ventrobasal complex includes the VPL nucleus (which receives somatosensory projections representative of the body) and the VPM nucleus (which receives the projections of the trigeminal area). The afferents come mostly from the nuclei of the posterior spinal cord and trigeminal complex, which run along the lemniscus medialis. The other areas projecting into these nuclei are: a) the marginal layer and nucleus proprius of the dorsal horns (and the corresponding area of the spinal nucleus of the trigeminal nerve), whose afferents reach the most caudal part of these nuclei, maintaining a somatotopic representation [
19,
20]; and b) the mesencephalic reticular substance [
35].
Stimulation of the dorsal portion of this complex, in awake humans, elicits non-painful tactile sensations [
36]. However, at the level of the caudal and ventral areas, localized pain sensations occur [
37]. On the contrary, lesions in the ventrocaudal region produce changes in pain sensitivity that can subsequently lead to hyperpathy [
30].
In summary, these VPM and VPL nuclei seem to be involved in the localization and identification of innocuous and noxious stimuli which are preferably conveyed through the lemniscal pathway.
6.2. Posterior Complex
The posterior complex comprises an ill-defined area between the medial geniculate body and the pulvinar nucleus. It receives afferents from the nuclei of the posterior spinal cord and the spinothalamic tract, as well as descending cortico-thalamic pathways of the somatosensory cortex. It projects to the retroinsular cortex.
Its stimulation produces unpleasant sensations. The function of this posterior complex in pain is still misunderstood and multiform, and some cells seem to be inhibited by the systemic administration of morphine [
38].
6.3. Intralaminar Nuclei
Intralaminar nuclei receive afferents from the RF as well as from the marginal layer of the spinal dorsal horn and the spinal nucleus of the trigeminal nerve. They receive virtually no projections from the lemniscal pathway. Like the latter complex, they receive ipsi- and contralateral information [
39]. Their stimulation causes diffuse unpleasant sensations and, sometimes, poorly localized pain. A lesion produced at this level can relieve incoercible pain [
30], and cells at this level can also be inhibited by the administration of morphine.
7. Cerebral Cortex
The cerebral cortex constitutes a higher level of the pain pathway, and three different somatosensory areas can be differentiated and highlighted (Figure 3).
7.1. Primary
The primary area corresponds to the postrolandic gyrus, including its medial extension into the paracentral lobe. It receives projections from the VPL and VPM nuclei. Stimulation produces well-localized sensations of touch and temperature, but rarely pain [
40]. Its removal may result in a loss of sensory-discriminative capacity, and sometimes improves pain, although hyperpathy may appear later [
41].
7.2. Secondary
Secondary areas are located at the level of the superior operculum of the Sylvian fissure, behind the fissure of Rolando and the retroinsular region. Many of the cells in these areas respond to noxious stimuli, and their stimulation causes localized pain sensations. There are reciprocal projections to the thalamus, VPM, VPL, and medial nuclei. Lesions at this level alter pain sensibility, not affecting the somatosensory-discriminative capacity [
42].
7.3. Tertiary
Tertiary areas correspond to the areas of the anterior cingulate and insula. They are related to the limbic system and visceral sensations. They connect with the medial thalamic nuclei and are related to the affective-emotional component of pain [
43].
Cortical resections have been abandoned because of their failure to relieve pain and subsequent complications. Only cingulotomy has remained as an analgesic intervention, although its effects on pain are due more to its action on personality and the affective component of suffering rather than on pain as a sensory activity [
41,
44]. The function of the cerebral cortex in the pain phenomenon remains elusive.
8. Pain Modulation Pathways
8.1. Neurophysiological
Experience indicates that chronic pain is rarely fully abated, rapidly or lastingly, after some type of surgical, physical, or behavioral intervention. Usually, after an appropriate regimen, patients might feel less pain and accordingly change their behavior in the long term, enabling them to lead a more productive life.
Numerous efforts have been made to explain this ability to modulate and modify pain. Melzack and Wall [
10] put forward a theory that persists today, although adapted, to explain these phenomena at the level of the first relay of the pain sensory system. The following provides a brief explanation of the gate control theory (
Figure 1 and
Figure 2):
At the spinal cord segmental level, there is a dynamic interaction between the input of information through the large myelin fibers (A) and that of thin (Aδ) and unmyelinated (C) fibers. This interaction takes place at the level of laminae II and III (substantia gelatinosa of Rolando [SGR]), where there are neurons that exert a presynaptic inhibition of both types of fibers at their synapses with T (transmitter) cells, presumably located in lamina V, from which the spinothalamic fibers arise directly or indirectly.
On the one hand, the large fibers send collaterals that excite the interneurons of the SGR, enhancing the inhibition of the T cells (the gate is closed, preventing the passage of pain information). On the other hand, the unmyelinated fibers send inhibitory information to SGR cells, reducing T cell inhibition (thus opening the gate, allowing the passage of pain information).
Melzack and Casey [
45] modified this behavioral approach with another theory, i.e., “central control trigger”, which implied that the information transmitted by the large fibers through the posterior spinal cord can activate central inhibitory mechanisms, whose downstream action modulates pain transmission by dorsal horn neurons. T cells can be inhibited or excited by information from the brainstem, which uses the posterior spinal cords as a transmission pathway [
21]. Melzack [
46], in 1975, further suggested that certain forms of neurostimulation activate a central biasing mechanism that inhibits chronic and pathological pain signals. This mechanism, with a neurophysiological or neurohumoral component, delays the pathological processes of fixation and correlation with old experiences, which occur simultaneously with the reception of the pain signal (in which the sensory inputs, inhibitory pathways of the brainstem, activity of the autonomic nervous system, individual’s expectations and anxiety, personality structure, etc., are combined).
However, just as the existence of a modulatory system at the level of the first relay in the spinal dorsal horn is becoming better known, there are, nevertheless, more complex and less understood modulatory systems located at higher levels of the CNS. As an example, we will only cite the fact that stimulation at the level of the VPL nucleus [
47] or the posterior limb of the internal capsule (thalamocortical pathway, of specific afferents) [
48] can produce analgesia. This phenomenon is explained by an inhibitory effect of these structures on other thalamic nuclei or the cortex [
35,
49]. This descending inhibitory system involves structures such as the ventrolateral prefrontal cortex [
50], the parahippocampal region [
51], or the amygdala [
52]. Therefore, chronic pain could be the result of a failure of inhibitory mechanisms rather than an excess of inputs [
53].
8.2. Neurochemistry
In 1975, Hughes et al. [
54] isolated two endogenous pentapeptides of morphinomimetic action: methionine and leucine–enkephalin. Subsequently, larger peptides, endorphins, with the same action and at many levels of the CNS were identified [
55,
56]. PAG and PVG are two areas with the highest abundance of endogenous opioid receptors, and in which the analgesia produced by their stimulation (SPA) has behavioral consequences similar to those obtained after the administration of morphine.
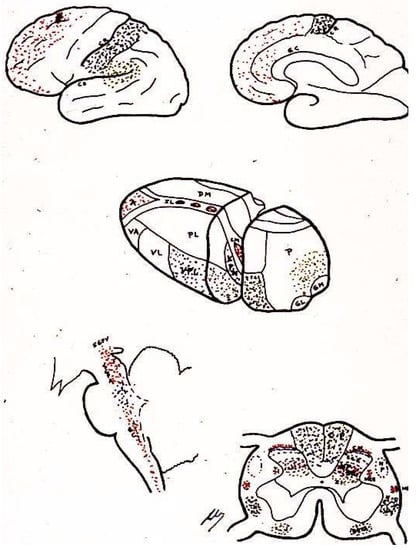
Enkephalins and endorphins have many potential places where they either modulate or serve as intermediaries by activating other systems (
Figure 4). As an example, there are enkephalinergic neurons at the level of the marginal zone and SGR; these same neurons also apparently receive many projections of primary nociceptive afferent terminals, whose neurotransmitter is substance P. Both morphine and enkephalins inhibit the release of substance P and reduce the response of neurons to the pain stimulation of their respective peripheral organs. Both morphine and enkephalins can act at this spinal cord level, activating a descending bulbospinal serotonergic system starting at the level of the raphe nuclei, which receive their information from the PAG. Both electrical stimulation of the PAG and administration of opioids at this level will produce the same analgesic effect, which can be blocked by the systemic administration of naloxone or by lesions, either at the level of the raphe nuclei or of the descending pathway in the dorsolateral fasciculus of Lissauer [
57].
Figure 4. Area distribution according to the neurotransmitters involved (see text). LD (Lateral dorsal nucleus). LP (Lateral posterior nucleus). VL (Ventral lateral nucleus). VA (Ventral anterior nucleus). VL = VPL (Ventral posterolateral). VP = VPM (Ventral posteromedial). A = AN (Anterior nuclear group). DM = MD (Medial dorsal nucleus). P (Pulvinar). GL = LG (Lateral geniculate nucleus).
However, the mechanisms are even more complex because, for example, the same nociceptive impulses can (through collaterals to the gigantocellular reticular nucleus and from here to the PAG) inhibit (via the lateral dorsal funiculus) the peripheral impulses reaching laminae II and V cells [
58]. Therefore, there are negative feedback mechanisms, which are mediated by endorphins and use serotonergic pathways.
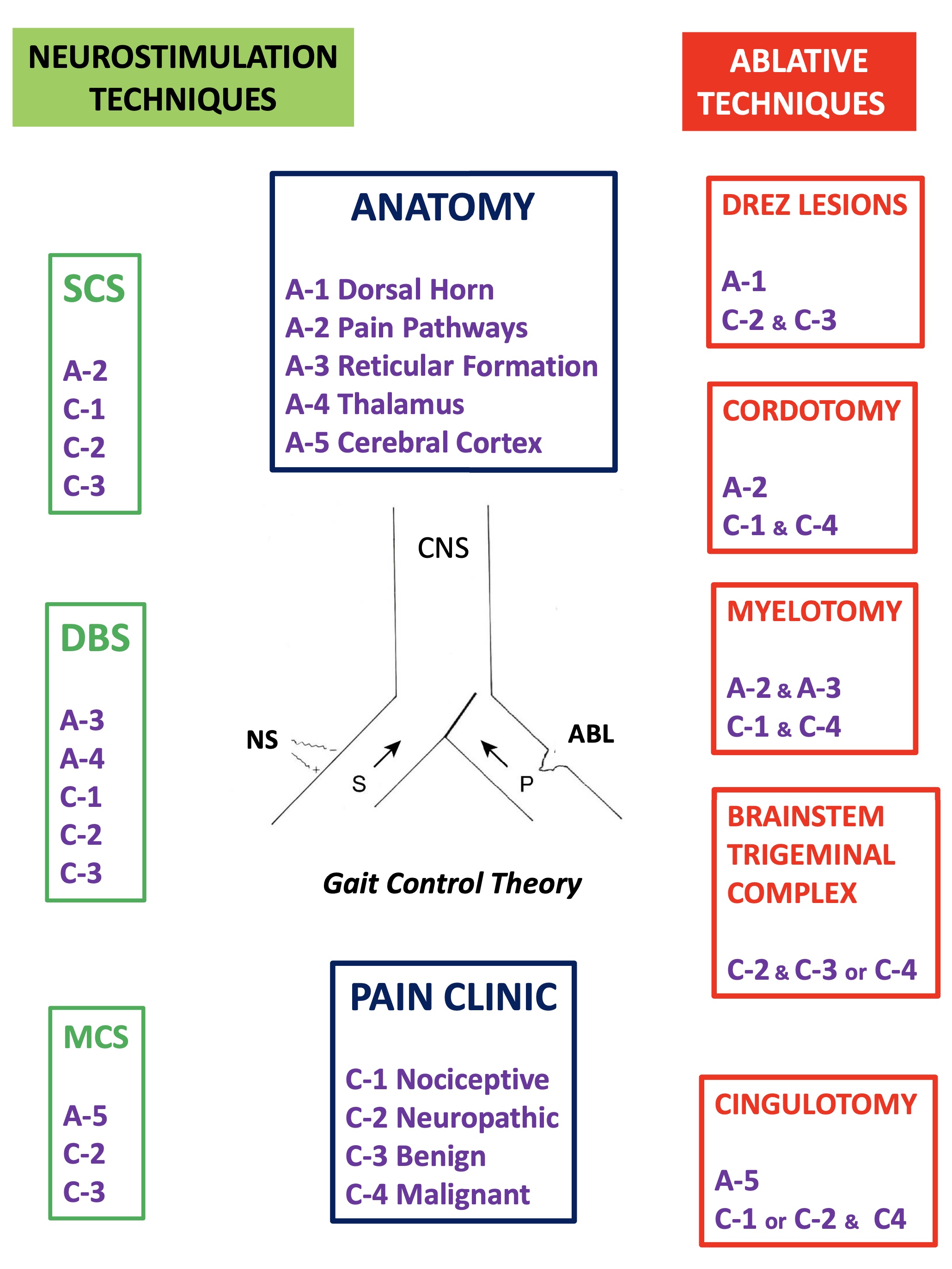
9. Gate Control Theory
Perhaps it would be better to have a simpler idea of what can occur at each relay of the pain pathway (dorsal horn, brainstem, thalamus…), helping to clearly explain the positive effects of surgical treatment.
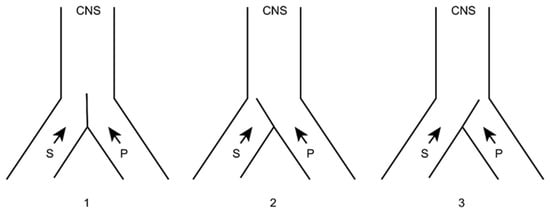
In this sense, we can imagine that both types of sensory (S) and pain (P) information arrive at higher brain levels (Figure 5). If, for any reason, the latter increases, the gate will turn to the left, preventing the passage of sensory information (we pay more attention to pain, i.e., to the nociceptive stimulus that has occurred).
Figure 5. Melzack and Wall gate control theory. 1: Normal. 2: Increased pain afferents. 3: Increased sensory afferents. (see text). CNS: Central Nervous System. S: Sensory afferents. P: Pain afferents.
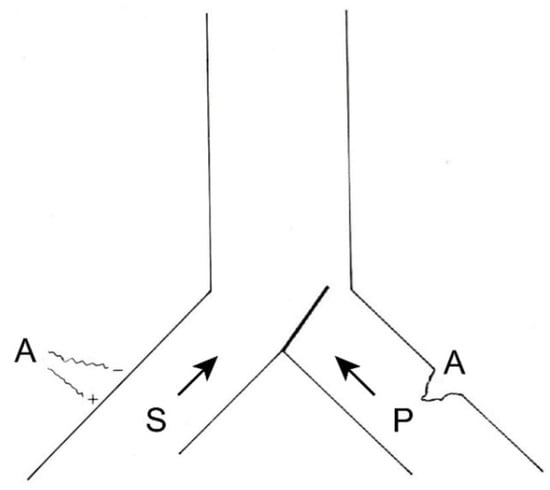
If this information were to remain constant and become chronic, we could reduce it in two ways (Figure 6), either by inhibiting the afferents of the same pain pathway (by pharmacological, instrumental, or even surgical means) or acting on the sensory pathway by increasing its afferents. The latter can be done in a natural way (for example, rubbing the skin after receiving a blow dulls the pain) or by means of neurostimulation.
Figure 6. Therapeutic possibilities for reducing pain afferents. On the right, surgical interruption of pain pathways; on the left, increasing sensory afferents through neurostimulation. (see text).
Another way of understanding the problem is the model proposed by De Ridder, et al. [
4] “in which pain (and suffering) is the consequence of an imbalance between the ascending and descending pain inhibitory pathways. This balance is theorized to be under control of the reward system”.