Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by ANTELM PUJOL CALAFAT and Version 3 by Jessie Wu.
Type 2 diabetes (T2DM) is an endocrine disorder that is characterized by hyperglycemia with alterations in carbohydrate, protein and fat metabolism. Microvascular and macrovascular complications are the main concerns in patients living with poor T2DM control. Phytate intake can play a role in controlling hyperglycemia but, more importantly, also in reducing cardiovascular risk by different mechanisms.
- phytate
- type 2 diabetes
- health
1. Background and In Vitro Studies
The 2022 American Diabetes Association Standards of Care focuses on treating type 2 diabetes (T2DM) with a metabolic-centric approach and not just a glucose-centric approach [1][81]. Most of the people living with diabetes mellitus (DM2) are patients with high or very high cardiovascular risk [2][82]. Controlling hyperglycemia and managing other cardiovascular risk factors would be the goal of the patient centered treatment [1][81].
Sustained and uncontrolled hyperglycemia produces changes in cell membrane permeability and transmembrane potential, influencing the relationship of the cell with the environment [3][83]. Thus, hyperpolarization induces glucose oxidation [4][84], protein glycation [5][6][85,86], activation of the polyol pathway, and increased oxidative stress, leading to a state of low-grade inflammation and pro-oxidative state [3][4][5][6][7][83,84,85,86,87].
Alterations in inositol metabolism (inosituria and inositol intracellular depletion) have been associated in several human and animal studies with hyperglycaemia and insulin resistance [8][88]. Phytate would reduce oxidative stress by acting as an iron chelator, preventing the generation of iron-driven hydroxyl radical formation and decreasing lipid peroxidation [9][10][11][72,73,74]. InsP6 and the formation of lowers forms (via degradation of InsP6) especially InsP3 play an important role in insulin secretion by regulating calcium-homeostasis [12][13][89,90]. Amylase inhibition activity by phytate has been described and would reduce the rate of carbohydrate digestion and absorption [14][91]. Moreover, phytate could exercise its positive effects too by decreasing leptin and increasing adiponectin levels [12][89]. On one hand, leptin action promotes an increase in the drive for food, reduced satiety, and energy utilization [8][88]. On the other hand, higher adiponectin concentrations produce an antioxidant response and are associated with a decreased level of C-reactive protein and interleukin-6 (IL-6) [8][88].
InsP6 influences lipid metabolism. Researchers have reported a reduction in lipase activity, total cholesterol, low-density lipoprotein, hepatic total lipids, and hepatic triglycerides, whereas increasing high-density lipoprotein levels are also seen in InsP6 supplementation [8][88]. The salt type of phytate administration will be crucial to the outcome lipid levels. The sodium-phytate form decreases cholesterol concentrations, whereas the calcium–magnesium form can increase cholesterol concentrations [8][88]. It is hypothesized that the calcium–magnesium form would not bind to bile acids, reducing fecal bile excretion [15][92].
Protein glycation leading to the accumulation of AGEs is thought to be one of the main factors triggering the diabetic complications, including nephropathy, retinopathy and neuropathy [15][16][17][18][19][20][92,93,94,95,96,97]. AGE accumulation alters the intracellular signalling and gene expression and releases pro-inflammatory molecules and free radicals [16][17][18][19][20][21][93,94,95,96,97,98]. In fact, proteins are not the only molecules that can produce AGEs as other endogenous components, lipids or nucleic acids can also lead to AGE formation [16][17][18][19][20][21][93,94,95,96,97,98]. Sanchis et al., 2018 [22][31], showed that InsP6 significantly reduces AGE formation, and in a dose-dependent manner, because InsP6 can strongly chelate Fe3+, preventing the subsequent formation of free radicals [22][31].
Red cell distribution width (RDW) is a numerical measure of the amount of variability in red blood cell size, which is routinely used in the differential diagnosis of anaemia and has been suggested as a predictor of cardiovascular diseases and anaemia [8][88]. Inflammation might increase RDW levels through the impairment of iron metabolism [23][99]. InsP6 could reduce RDW through anti-inflammatory and antioxidant effects [8][88].
Hyperuricemia it is considered a risk factor for cardiovascular events, and it is the most frequent cause of acute arthritis in men [24][100]. Even countries where the local population had historically low levels of serum uric acid, have experienced an increase in serum uric acid concentration (SUA) due to the acquisition of a Western diet eating pattern [24][100]. Restricting dietary purine intake should be an effective method for maintaining fasting SUA. However, restricting purine intake in the long term could be difficult [24][100]. InsP6 has been shown to inhibit purine metabolism in vitro by competitively inhibiting the hydrolysis of purine nucleotides [24][100]. An overview of the potential cardiovascular bioactivities of phytate can be seen below (Figure 1).
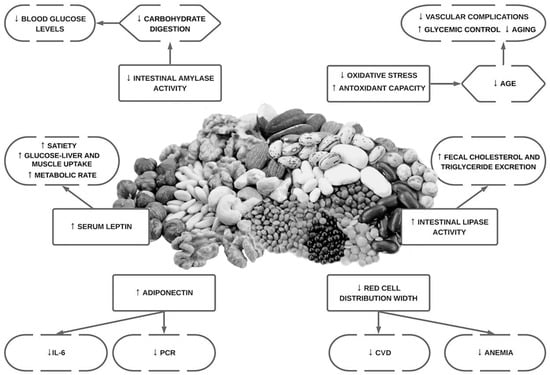
Figure 1.
Overview of the potential bioactivities in cardiovascular health of phytate.
2. Animal Studies
Dilworth et al., 2005 [12][89], in a comparative study in rats that were fed phytate plus zinc, phytate alone, zinc alone or placebo, the activities of enzymes involved in carbohydrate and lipid metabolism, as well as transaminases in the liver were assessed. The phytate came from two different sources: phytic acid extracted from sweet potato (Ipomea batatas) or commercial phytic acid. Phytic acid lowered blood glucose (seen with both phytate sources) and increased the activity of glucose-6-phosphate dehydrogenase (this was only seen with phytic acid extracted from sweet potatoes). NADPH generation by glucose-6-phosphate dehydrogenase was used by glutathione reductase to maintain reduced glutathione levels. The promotion of an antioxidant environment could be one of the explanations as to why the up-regulation of the glucose-6-phosphate dehydrogenase activity by phytate could reduce insulin resistance [7][87]. Similar results were previously found by Onomi et al., 2004 [25][101], in rats fed a high-sucrose diet. A high-sucrose diet and an amount of phytate ranging from 0.2% to 10% were provided [25][101]. Rats who ingested 10% sodium phytate experienced a reduction in lipogenic enzymes and lower growth, food intake, serum triglyceride and cholesterol levels, in a dose dependent manner [25][101]. Lee et al., 2006 [26][102], reported that diabetic KK mice who were fed purified diets supplemented with different concentrations of sodium phytate (0, 0.5 and 1%) over eight weeks reduced calorie intake, body weight, levels of fasting and random blood glucose, glycated hemoglobin (HbA1c) as well as insulin levels [26][102].
Phytate can cause inhibition, in a dose-dependent manner, of α-glucosidase and α-amylase activity comparable to standard drug acarbose, in vitro and in rats with streptozotocin–nicotinamide-induced type 2 diabetes mellitus [27][103]. The antidiabetic function of phytic acid may work in part through the decrease in the activity of intestinal amylase which is indicative of lesser products of carbohydrate digestion formation and subsequently absorption, leading to a decreased percentage spike in random blood glucose [27][28][103,104].
A high-fat diet and streptozotocin is a model used to induce T2DM in Sprague Dawley rats. Omoury et al., 2013 [28][104] used this model to study the effects of the combination of InsP6 and inositol, in comparation with glibenclamide, on several markers of metabolic health over four weeks. The combination of InsP6 and inositol in the mentioned dose achieved better glycemic control (reduced blood glucose plus reduce HOMA-IR index) than glibenclamide. Interestingly, this was the first study to show that leptin levels were increased by phytate [28][104]. The increased concentration of leptin can explain why, in this study, the rats treated with phytates reduced their food intake by 45%. The effects of phytate intake on lipid metabolism were as expected: reduction in triglycerides and total cholesterol as seen in various works [25][28][101,104]. A later study [29][105], using a very similar approach, found that the increase in serum α-amylase activity in diabetic rats treated with combined InsP6 and inositol or glibenclamide was not significant compared with (that of) the nondiabetic control group. Low serum α-amylase concentrations are associated with pancreatic exocrine-endocrine disorders. The authors hypothesized that the nonsignificant increase in serum α-amylase activity in diabetic rats treated with combined InsP6 and inositol or glibenclamide, compared with the nondiabetic control group, might restore the metabolic abnormalities caused by T2DM. A decreasing trend in the Na+/K+ ATPase activity in the group treated with combined InsP6 and inositol supplement could reduce intestinal carbohydrate absorption. Moreover, in this later study, the authors found that a reduction in RDW levels in the diabetic rats treated with the InsP6 and inositol or glibenclamide can reduce cardiovascular risk.
3. Epidemiological Studies in Humans
Mediterranean and DASH diets have repeatedly shown their effectiveness in improving glycemic control and decreasing cardiovascular risk. Furthermore, these diets are rich in phytates [30][31][33,34]. In the latest “Dietary Guidance to Improve Cardiovascular Health” the Mediterranean diet and the DASH diet are recommended to reduce cardiovascular disease risk (CVD) [32][24]. The ATTICA study [33][106] revealed that those who had a higher adherence to a Mediterranean diet improved fasting glucose homeostasis, insulin levels and a lower insulin resistance index (HOMA) in both normoglycemic and diabetic patients [33][106]. Several works proved that adherence to the Mediterranean diet exerts a protective effect against loss of glycaemic control [33][106]. These effects on cardiovascular health and glycemic control could be produced by the anti-oxidation and anti-inflammation effects of the Mediterranean diet [34][107].
In a more specific way, certain foods present in the Mediterranean diet have been investigated in isolation. Several studies proved that a higher intake of legumes and nuts for cardiovascular prevention [32][35][36][24,25,26]. Nuts are identified as a protective factor against cardiovascular disease, especially coronary heart disease and stroke incidence and mortality [36][37][22,26]. Improvements in glycaemic control and a reduction in HbA1c were reported as a result of increased dietary intake of legumes and wholegrains [34][38][39][107,108,109]. High intake of dietary fibre, specifically of the soluble type, improves glycaemic control, decreases hyperinsulinemia, and lowers plasma lipid concentrations in patients with type 2 diabetes [38][108].
Legumes and whole grains are rich in InsP6 and in fiber; this could explain, to a certain extent, the benefits reported in the scientific literature. Sanchis et al., 2018 [22][31], mentioned that observational evidence suggests that in the Mediterranean region, the InsP6 consumption is lower in patients with T2DM than in non-diabetic subjects (unpublished data) [22][31].
4. Clinical Trials in Humans
Most clinicals trials published in this regard investigate the effects of the Mediterranean diet or DASH diet on T2DM or cardiovascular health. The multi-centre, randomized, primary prevention trial of cardiovascular disease (Prevención con Dieta Mediterránea “PREDIMED” Study) [40][110] in 772 asymptomatic patients, aged between 55 and 80 years of age at high cardiovascular risk, saw improved fasting blood glucose, reduced blood pressure and increased high-density lipoprotein (HDL)/cholesterol ratios in these diets in comparison with a low-fat diet. The participants did not lose weight on either diet [40][110]. Toobert et al., 2013 [41][111] tested the effectiveness of the Mediterranean Lifestyle Program (MLP) in 279 post-menopausal women. After six months of intervention a reduction in 0.4% units in HbA1c was reported [41][111]. In a one-year randomized trial of 259 patients people living with T2DM, a low-carbohydrate Mediterranean diet, a traditional Mediterranean diet, and an American Diabetes Association (ADA)-proposed diet were compared. The low-carb Mediterranean diet and the traditional Mediterranean diet showed better weight loss effects and better reduction in HbA1c compared with the other diet [42][112].
The effectiveness of certain high-phytate foods in improving cardiovascular health has been tested in clinical trials. Recently, 31 people living with T2DM were randomly assigned to two different groups: one designated to consume a legume-free diet, the other to consume a legume-based diet for 8 weeks. Legumes significantly increased serum adiponectin concentrations [43][113].
Because it is difficult to elucidate or discriminate the effects of phytate from the other components of the diet, clinicals trials using only InsP6 are much needed. In fact, Sanchis et al., 2022 [44][114] in a randomized crossover trial they provided to people living with T2DM 1 capsule of 380 mg of calcium-magnesium InsP6 twice daily during 12 weeks. When patients received InsP6 supplementation they had significant decrease serum levels of HbA1c and increase adiponectin levels. However, no differences were found in IL-1beta, IL-6 and tumor necrosis tumor necrosis alpha (TNF-alpha). This work proves for the first time that phytate supplementation could increase adiponectin levels in patients living with T2DM.
Sanchis et al., 2018 [22][31] for the first time reported the inhibitory effect of InsP6 on protein glycation, reducing both in vitro and in vivo AGEs in patients living with T2DM. In this randomized cross-over trial [22][31], 35 patients received either an InsP6 diet (diet plan plus one capsule of 380mg of calcium-magnesium InsP6) or a non-InsP6 diet (the same diet plan without InsP6 supplementation). When the subjects took the InsP6 supplementation, they experienced a reduction of 25% of the levels of circulating AGEs and a 3.8% decline in HbA1c, probably because of reduced overall protein glycation.
Ikenaga et al., 2019 [24][100] in a randomized double-blind placebo-controlled trial assessed the effect of the repeated intake of InsP6 on fasting SUA levels in hyperuricemic subjects, and demonstrated that two weeks of supplementation with twice-daily 600 mg of InsP6 improved fasting SUA levels in these subjects [24][100].
