Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ligeng Ma and Version 2 by Camila Xu.
In flowering plants, pollen development is a key process that is essential for sexual reproduction and seed set. The tapetal cells secrete nutrients, proteins, lipids, and enzymes for microsporocytes and microspore development, while initiating programmed cell death to provide critical materials for pollen wall formation in the late stage.
- pollen development
- tapetum specification
- tapetum function
1. Introduction
In higher plants, the alternation of the life cycle mostly depends on sexual reproduction, in which normal pollen development is necessary. In the past decades, extensive studies have shown that pollen development is a complex biological process, which includes anther primordial cell differentiation, pollen mother cell meiosis, microspore mitosis, tapetal cell differentiation and programmed cell death, pollen wall formation and pollen grain release [1][2][1,2]. According to the morphological properties, anther development was divided into 14 stages by Sanders et al. [3]. Molecular analysis has demonstrated that a great number of genes are expressed and function during male reproductive development, and the proteins encoded by these genes are involved in transcriptional regulation, protein degradation, hormone biosynthesis, and signal transduction [2][4][2,4]. In addition, these studies on pollen development are also important for crop yield, crop breeding, and plant propagation.
Over the past two decades, extensive studies indicated that pollen development is coordinately controlled not only by gametophytic but also by sporophytic regulators, especially the tapetum for the latter case [5][6][7][5,6,7]. The tapetum is the innermost cell layer of the anther wall, which is in direct contact with the developing microsporocytes [8][9][8,9]. In general, the development of tapetal cells is initiated at floral stage 8/anther stage 4 and can be divided into three stages, including tapetum differentiation, cell binucleation, and tapetum programmed cell death [1][3][1,3]. Molecular genetic studies have discovered that tapetal cells play a vital role in pollen development by secreting nutrients, enzymes for microsporogenesis at the early stage, synthesizing and secreting callose-degrading enzymes to release microspores from tetrads, and initiating programmed cell death to provide nutrients and signals for developing microspores, multiple materials for pollen wall formation [5][7][10][5,7,10]. Therefore, once the tapetum develops defectively, it usually leads to serious defects in male fertility. An array of genes required for pollen development have been defined and summarized in many excellent reviews in recent years [5][6][10][11][5,6,10,11].
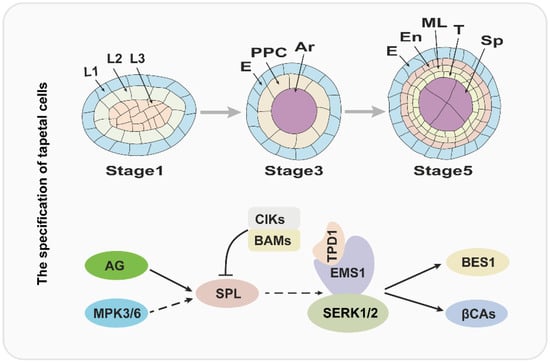
2. The Specification of Tapetal Cells Is Essential for Pollen Development
In flowering plants, pollen development is critical for sexual reproduction and usually occurs in the special tissue: the anther. More importantly, the pollen and anther development are coordinated and precisely regulated. According to the morphological characteristics, anther development has been divided into 14 stages in Arabidopsis [3]. At stage 1, anther primordium emerges from the floral meristem that consists of three cell layers (L1–L3) [1][4][1,4]. Later, the outside L1 layer develops to form the epidermis of the anther by anticlinal cell division, and L3 layer cells divide and differentiate to form the connective and vascular tissues; the L2 layer cells undergo an array of divisions to form sporogenous cells and three maternal cell layers, comprising the endothecium, middle cell layer and tapetum from outside to inside [1][4][7][12][1,4,7,12]. Over the past several years, molecular and genetic studies have uncovered that the development of anthers is regulated by BCE genes of the well-known ABCE model cooperatively [6]. In Arabidopsis, B-class genes APETELA3 (AP3) and PISTILLATA (PI), C-class gene AGAMOUS (AG), and E-class genes SEPALLATA1/2/3/4 (SEP1/2/3/4) play a critical role in anther identity [4][6][4,6]. Therefore, the mutation of AP3, PI, AG, or SEP1/2/3/4 usually causes serious anther developmental defects. The SPOROCYTELESS/NOZZLE (SPL/NZZ) gene encodes a MADS-box transcription factor and is expressed in the L2 layer; and it is activated by AG protein and functions in the specification of reproductive cells [13][14][15][16][13,14,15,16]. Anthers of spl/nzz contain primary parietal, primary sporogenous cells, and normal initiated archesporial cells, but the pollen mother cells (PMCs) cannot be formed and the tapetum development is also defective, suggesting that the SPL/NZZ is essential for early anther development [13][16][13,16]. A recent investigation revealed that the SPL/NZZ can interact with and be phosphorylated by MITOGEN-ACTIVATED PROTEIN KINASE 3 (MPK3) and MPK6, which enhance the stability of SPL/NZZ [17]. In addition, the BARELY ANY MERISTEM 1 and 2 (BAM1 and BAM2), which encode two homologous and functional redundancy leucine-rich repeat receptor-like protein kinases (LRR-RLKs), are reported to interact with and suppress the expression of SPL/NZZ [18]. In bam1bam2 anther, the cell division and specification of L2 derived are disordered, which leads to a lack of the endothecium, middle layer, and tapetum, in contrast to generating excess pollen mother-like cells [18]. Similar to BAM1/2, anther LRR-RLK, RECEPTOR-LIKE PROTEIN KINASE2 (RPK2) also plays an important role in anther lobe identity and early anther cell specification [19]. Owing to the disordered cell differentiation, anthers in rpk2 lack the middle layer and exhibit hypertrophic tapetum and defective endothecium, which ultimately results in the males being sterile [19][20][19,20]. It is widely accepted that the ligand binding of RLKs usually functions in signal transduction by recruiting other RLKs as co-receptors [21]. A recent report has shown that a group of novel RLK proteins CLAVATA3 INSENSITIVE RECEPTOR KINIASEs (CIKs) functions as coreceptors of RPK2 and BAM1/2 to participate in the regulation of archesporial cell division and parietal cell specification in early anther development [20]. The anther phenotypes of these CIKs gene mutants are similar to the bam1bam2 and rpk2, including a lack of one to three parietal cell layers and excess microsporocytes [20]. In short, this evidence revealed that CIKs function with BAM1/2 and RPK2 in the same pathway during the early anther development, but the ligand signals and substrates of the BAM1/2-CIK and RPK2–CIK complex remain to be explored in the future. Tapetum is the most inner somatic cell layer of the anther and is adjacent to the developing microsporocyte and/or microspores directly [12]. More importantly, during the anther development, the tapetal cells can provide abundant nutrition, lipide, and enzymes for gametogenesis by the way of secreting or programmed cell death [5]. Defects in tapetum specification and development result in failed pollen development and impaired fertility [22][23][24][22,23,24]. Molecular genetic studies have revealed that the regulation mechanism of tapetal specification and development is a complex network [12][25][12,25]. Over the past two decades, several studies suggested that the specification of the tapetum is mainly regulated by the TPD-EMS1-SERK1/2 signaling pathway in early anther development [23][26][27][28][29][30][31][32][23,26,27,28,29,30,31,32]. The EXCESS MICROSPOROCYTES1 (EMS1), which is also named EXTRA SPOROGENOUS CDLLS (EXS), encodes an LRR-RLK and is expressed in both sporogenous and parietal cells in early anther, especially, strongly expressed in tapetum, suggesting that EMS1/EXS is associated with microsporocytes and tapetal cells differentiation [26][27][26,27]. When the function of EMS1 is disrupted, the anther has no tapetal layer, but instead presents excess microsporocytes [26][27][26,27]. Cytological observation showed that these excess microsporocytes can undergo normal meiotic nuclear division, but cytokinesis fails to occur, leading to unsuccessful microsporogenesis and male sterility [27]. As an LRR-RLK, EMS1 kinase activity relies on its autophosphorylation status [30]. A recent study uncovered that EMS1 can interact with and be transphosphorylated by SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 (SERK1), which is fundamental for enhancing EMS1 kinase activity [32]. The SERK1 also encodes an LRR-RLK protein and redundantly regulates the tapetum development with its homologous protein SERK2 [28][29][28,29]. The SERK1 and SERK2 share 90% identity in the primary amino acid sequence; therefore, neither the serk1 nor serk2 display obvious anther defects [28][29][28,29]. Similar to the ems1, the anthers of serk1 serk2 lack the tapetal layer and produce more pollen mother cells, which suggests that SERK1/2 function with EMS1/EXS in a common pathway [28][29][32][28,29,32]. In addition, the TAPETUM DETERMINANT1 (TPD1) was proven to function in tapetum specification, and indistinguishable phenotype compared with ems1 and serk1serk2 was observed in tpd1 in early anther development [23]. TPD1 encodes a small cysteine-rich protein with 176 amino acids, which is secreted from reproductive cells [23][31][23,31]. Furthermore, molecular studies showed that TPD1 works as the ligand to interact with tapetum precursor plasma membrane localized-EMS1 to activate EMS1 phosphorylation and then determine the tapetal cells’ fate [23][30][31][33][23,30,31,33]. Taken together, the tapetum specification is regulated by the TPD1-EMS1-SERK1/2 signal pathway, in which the TPD1 is the ligand, which is recognized by EMS1, and SERK1/2 may be a coreceptor of EMS1. Considering the critical role of the TPD1-EMS-SERK1/2 signaling pathway in tapetum specification, it is important to uncover its downstream factors. Recently, β-CARBONIC ANHYDRASES (βCAs) and BRI EMS SUPPRESSOR 1 (BES1) family members as the downstream factors of the TPD1-EMS1-SNRK1/2 signaling pathway were identified [34][35][34,35]. In Arabidopsis, it is clear that βCAs play a vital role in photosynthesis through concentrating CO2. There are six members in the βCAs family (βCA1 to βCA6), among which βCA1, βCA2, and βCA4 have been demonstrated to interact with EMS1, suggesting that βCA1, βCA2 and βCA4 function with EMS1 in the same pathway [35][36][35,36]. Indeed, the βca1βca2βca4 shows defects in tapetal cell differentiation and tetrad formation [35]. Moreover, βCA1, βCA2, and βCA4 can be phosphorylated by EMS1 and the phosphorylation of βCA1 leads to its activity being enhanced significantly [35]. Consistent with this observation, the phosphorylation blocking mutations of βCA1 cannot rescue the phenotype of βca1βca2βca4, while the phosphorylation mimic mutation is able to form tapetum [35]. The BES1 family members (BES1, BZR1, BEH1, BEH2, BEH3, BEH4), especially BES1 and BZR1, as key transcription factors, mediate an array of gene expression in the BR signaling pathway [34][37][38][34,37,38]. However, Chen et al. demonstrated that BES1 family members regulate tapetum development by acting as the downstream factors of the TPD1-EMS1-SERK1/2 signaling pathway [34]. The quintuple mutant, bes1bzr1beh1beh3beh4, fails to develop the tapetal cell layer and microspores, similar to ems1, tpd1, and serk1/2 [34]. And the gain-of-function mutation of BES1 or BZR1 can partially rescue the phenotype of ems1, tpd1, and serk1/2. Remarkably, the expression of TPD1 or EMS1 driven by the BRI1 promoter in bri1-116, a BR receptor knock-out mutant, significantly leads to the accumulation of non-phosphorylated, active BES1, which indicates that BES1 regulates tapetal development in a BR signaling-independent manner [34]. The molecular model is summarized in Figure 1.
Figure 1. The regulatory pathway for the tapetum specification. The upper panel of the figure is a diagram of tapetum differentiation, and the lower panel is the corresponding regulatory pathway. L1, L2, L3: three cell layers of anther primordia. E: epidermis; PPC: primary parietal cell; Ar: archesporial cell. En: endothecium; ML: middle layer; T: tapetum; Sp: sporogenous cell.
