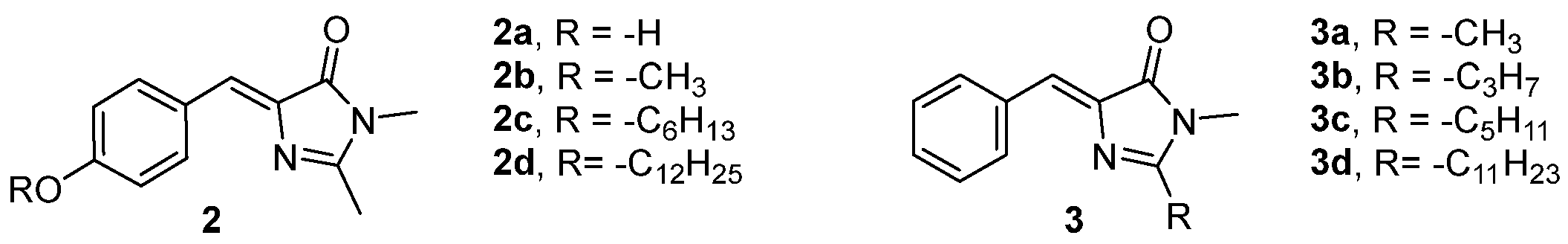Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Dean Liu and Version 2 by Dean Liu.
The Green Fluorescent Protein (GFP) and its analogues have been widely used as fluorescent biomarkers in cell biology. Yet, the chromophore responsible for the fluorescence of the GFP is not emissive when isolated in solution, outside the protein environment. Many efforts have been devoted to the study of this famiy of fluorophores, especially on the ways to restaure their emission intensity without modifying their backbone. Here are presented several ways to enhance the emission intensity of these fluorophores, modifying their environment but not their structure.
- fluorescence
- green fluorescent protein
- Z/E isomerization
1. Crystallization and Aggregation-Induced Emission Enhancement (AIEE)
The internal rotations of a chromophore can be blocked when it is in the solid state, as a crystal or an amorphous aggregate, which can lead to the enhancement or induction of the emission, in chromophores that are poorly- or non-fluorescent in solution.
A family of GFPc analogues has been synthesized using Erlenmeyer azlactone synthesis, followed by a reaction of the obtained oxazolones with p-anisidine to give origin to the benzamides ring opening with a final cyclization (chromophores 1a-c, Figure 1).[[1] The strategy is based on the introduction of rotational aromatic groups around the imidazolidinone core to increase the AIEE phenomena. In solution, the synthesized chromophores present weak emission, due to the relaxation through the Z/E isomerization and torsional vibration of the aromatic rings. This isomerization could be blocked in the aggregate or crystalline state, from where the emission was increased, as expected, and only the presence of the Z isomer was confirmed through the crystal structures.
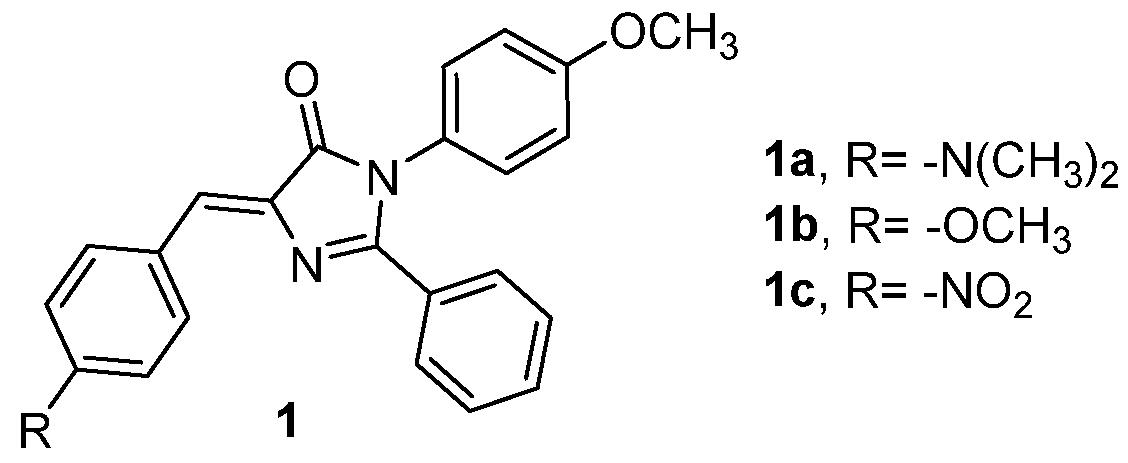
Figure 1. GFPc analogues with multiple phenyl substituents.[1]
The crystal packing of a chromophore is considered an important factor to achieve an enhanced emission. The length of the chain attached to the phenolic oxygen influences the crystal packing and the emission properties of the four GFPc derivatives 2 (Figure 2).[2] Crystals of chromophores 2b, 2c and 2d present AIEE properties. The emission of these chromophores increases along with the alkyl chain length, due to the reduction in the interaction strength between the molecules. The chromophore 2a did not show fluorescence emission even in the solid state, probably due to the intermolecular hydrogen bonds between the hydroxyl and carbonyl groups.
The presence of an alkyl chain on the position 2 of the imidazolinone ring (chromophores2a-d, Figure 2), demonstrates an effect similar to that described above. Regardless of the length of the alkyl chain, all derivatives are weakly emissive when in solution, but their emission intensity increases when they are in the solid state.[3] Additionally, the solid-state quantum yields increase with the extension of the alkyl chain. This phenomenon could be explained by the changes in the intermolecular arrangements and interactions, which results in weakened intermolecular π–π interactions between the benzylidene–imidazolinone moieties.
A family of GFPc containing a 2-phenylbenzoxazole group, and different alkyl chains on the nitrogen in position 3 of the imidazole ring (chromophores 3a-f, Figure 3) were described as weakly fluorescent in solution, with quantum yields around 0.02, with the exception of 3e and 3f which exhibit quantum yields of 0.20 and 0.24, respectively.[4][5] The quantum yield of 3a does not increase in the crystalline state, while all the other show an AIEE effect with quantum yield of 0.16–0.26 for 3b-d in the solid state. Derivatives 3e-f demonstrate a bright fluorescence when adsorbed on a solid support, such as nylon or paper, with a red-shifted wavelength relatively to the DMSO solution, mainly due to the stiffening of the molecules by the adsorption on the solid material.[5]
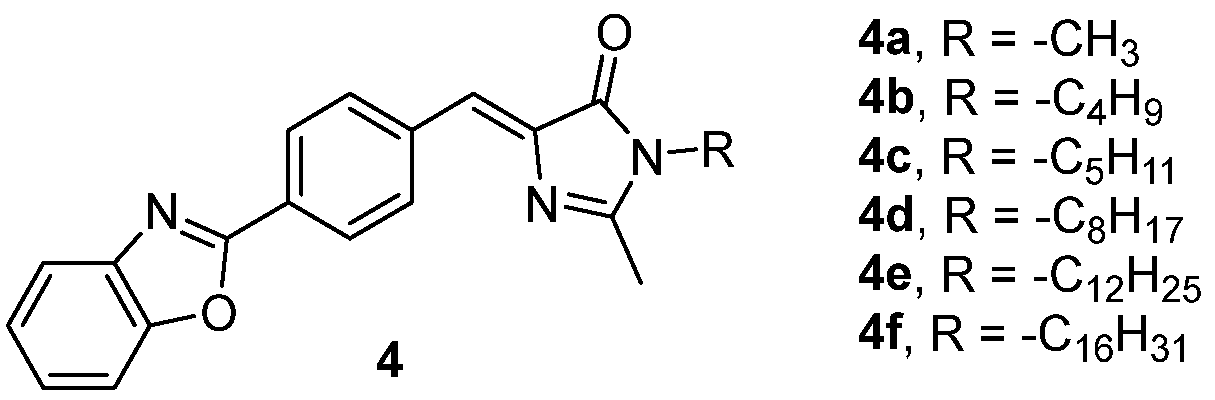
The different behaviors can be rationalized considering the crystal packing of the dyes. The crystal structure of 4a reveals that it arranges into head-to-tail dimeric structures, with a strong overlap of the benzoxazole of one molecule with the imidazole ring of the other molecule, probably quenching the emission. This overlap is much weaker for the remaining derivatives, preventing the quenching and allowing the increase in their fluorescence through the AIEE effect. Additionally, the long alkyl chains, in the case of 4e-f, seem to increase the fluorescence intensity by separating the molecules, which is in agreement with the other results.[3]
In another article, the polymorphism of 4a was studied, and the different polymorphs demonstrated to have different photophysical properties.[6] Some polymorphs are non-emissive, but two of them, one containing the pure dye and one solvate, exhibit a strong emission at 502 nm (φ = 0.22) and 582 nm (φ = 0.11), respectively. Molecular motions are restricted in all polymorphs; therefore, all should present AIEE properties, but in some of them, the strong overlap of aromatic rings probably counteracts the AIEE effect with an ACQ effect, quenching the emission.
The emission of the GFPc 4 (Figure 4) was not detected in solution. In the crystalline state, 4 can organize in five different polymorphs, all of them presenting AIEE properties with quantum yields of 0.02-0.05, due to the restriction of molecular motions. The emission wavelength is different between the polymorphs, varying from a blue emission around 450 nm to a yellow emission around 550nm, as a result of the strength of the π–π interactions between the donor and the acceptor units in the polymorphs.[7]
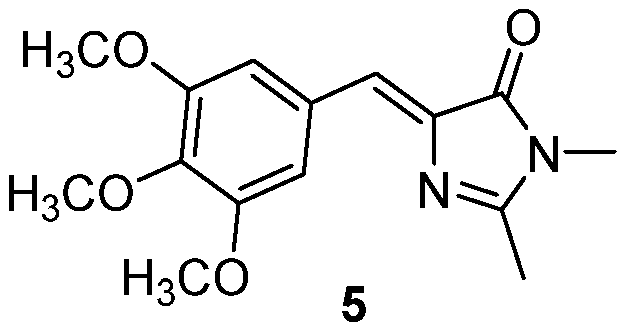
Figure 4. GFPc analogue emitting in the solid state, at different wavelengths depending on the polymorph.[7]
In an attempt to rigidify the backbone of the dyes, large aromatic substituents were introduced in replacement of the p-hydroxyphenyl ring on the GFPc 6a-e (Figure 5).[8] Unfortunately, this did not restrict the intramolecular motions in solution and, consequently, did not increase the emission intensity. However, these modifications induced a red shift of the emission wavelength due to extra π conjugation, and solvent polarity dependence. This shift was also observed in the solid state, but GFPc 6a, 6c and 6d do not present AIEE properties, being only faintly emissive. On the contrary, 6b crystallized in two polymorphic forms, one of them strongly emissive.
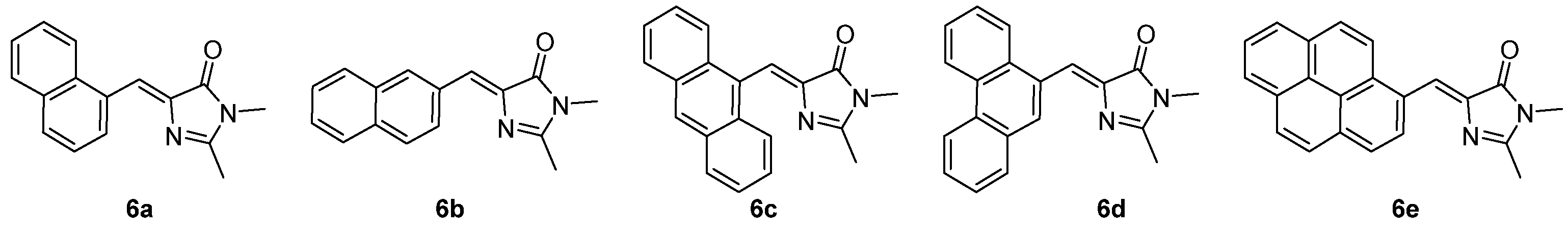
Figure 5. GFPc analogues with large aromatic substituents.[8]
The introduction of a diphenylmethylene group in GFPc 7a,b (Figure 6) was expected to enhance their emission intensity in solution, because it would suppress the possibility of Z/E isomerization (the virtual Z and E isomers are the same molecule). However, they are only faintly emissive in solution. Nevertheless, GFPc 7 presents AIEE properties in the solid state and in frozen solution, due to the restrictions of molecular motions. The crystal packing of GFPc 7a revealed intermolecular π–π interactions, responsible for the quenched fluorescence.[9]
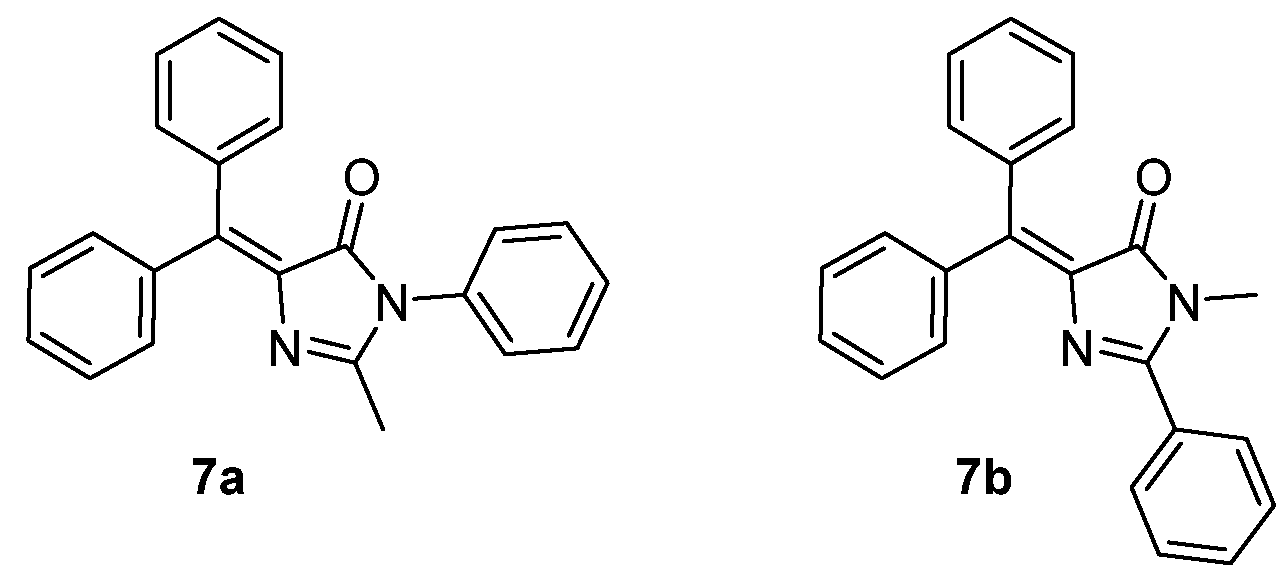
Figure 6. GFPc analogues without Z /E isomerization.[9]
Looking at these examples, wresearche rs can conclude that many GFPc analogues present AIEE properties and are brightly emissive in the solid state. However, many others are not, and it is difficult to predict the photophysical properties of a GFPc analogue in the solid state. Nevertheless, this approach is limited to the use of the luminescent dyes in the solid state, and many applications require the dyes to be luminescent in solution.
2. Supramolecular Hosts
In the GFP, the GFPc is surrounded by the β-barrel, which restricts its torsional vibrations and hinders the isomerization of the phenylene double bond, therefore producing the same effect as aggregation, but preventing ACQ as the dye is isolated. Following a biomimetic approach, supramolecular hosts have been used to encapsulate some GFPc analogues, in an attempt to enhance their emission intensity.
It has been demonstrated that some GFPc analogues (chromophores 8, Figure 7) could serve as guests inside a deep cavity cavitand (called “octaacid”). These dyes are poorly emissive in solution, but their emission intensity increased dramatically when they enter the cavity of “octaacid”. The GFPc 8k, bearing the longest alkyl substituent, was the one that demonstrated the greater increase in emission quantum yield, from 1.47% in benzene to 10% in the presence of “octaacid”.[10]
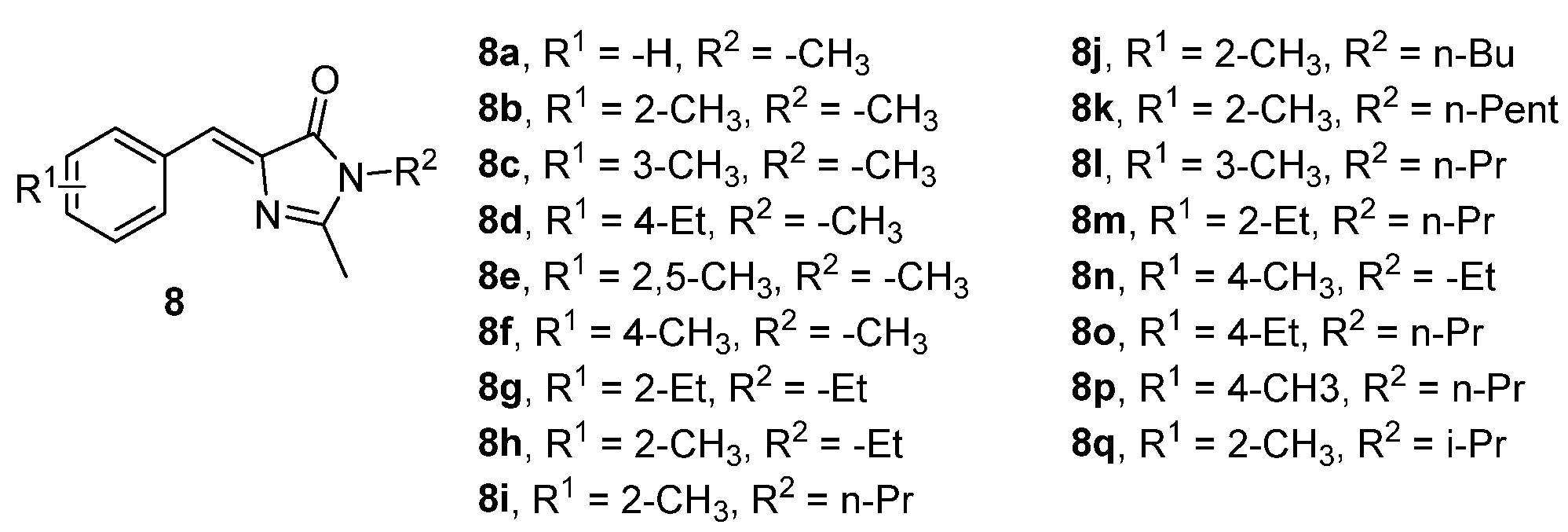
Figure 7. GFPc analogues demonstrating an increased emission intensity in the presence of a deep cavity cavitand.[10]
The fluorescence of a series of GFPc derivatives have been studied in the presence of different biomacromolecules, and these dyes demonstrated that these could be used as small molecular probes (chromophores 9a,b, Figure 8). The presence of an alkyloxy group in the para position of the phenyl ring induced a turn-on fluorescence when in the presence of ribonucleic acid (RNA), while the diethylamino group in the same position induced a selective fluorescence increase in the presence of human serum albumin (HSA). It is likely that the interaction of the dyes with the biomacromolecules stiffen their backbone, thus restricting molecular motions and isomerization.[11]
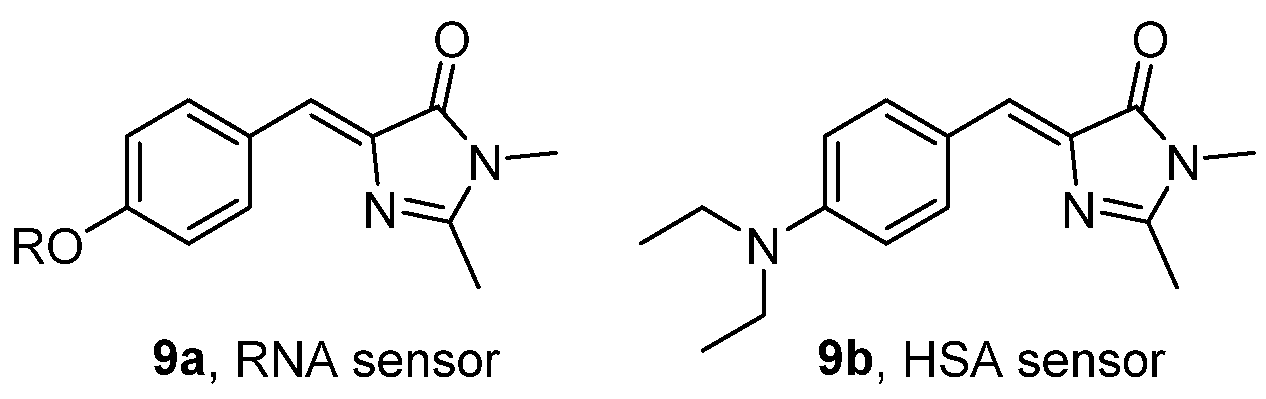
Figure 8. GFPc analogues used as selective fluorescence turn-on molecular probes for RNA and HSA.[11]
Moreover, all GFPc 10 (Figure 9) demonstrated that their fluorescence intensity increases when bonded to HAS; the longer the alkyl chain of R2, the more dramatic the enhancement, with chromophore 10k being the one that showed the best results.[12]
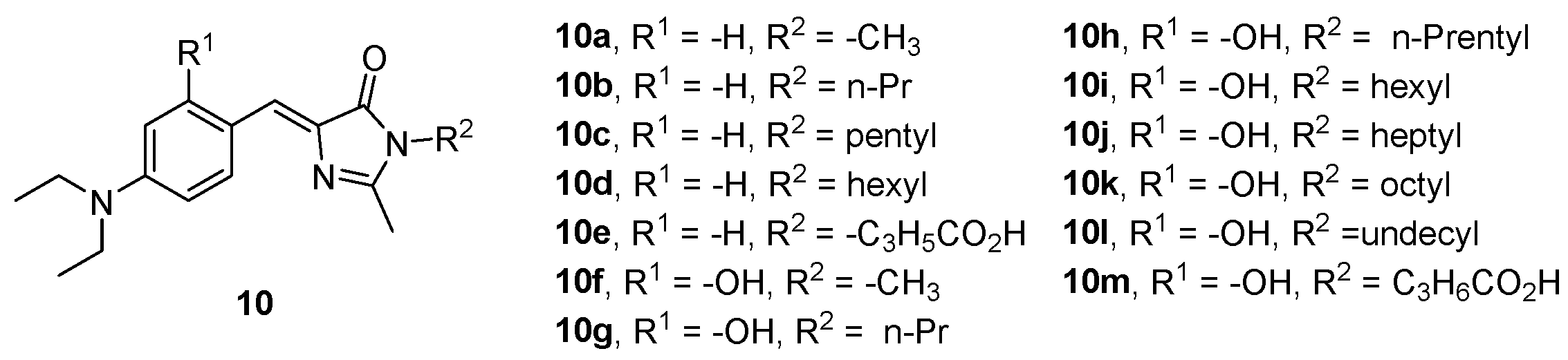
Figure 9. GFPc analogues with enhanced emission intensity in the presence of HSA.[12]
Similar analogues of the GFPc (chromophores 11, Figure 10) also demonstrated an enhanced emission intensity when encapsulated inside amphiphilic sodium cholate (NaCh), most probably because their torsional motions are restricted in the aggregate cavity. The emission intensity further increases alongside the concentration of NaCh. The authors of this study also proposed that the groups o-CF3 and n-Pr promote the optimal packing in the aggregate cavity, and an additional hydrophobicity, respectively, which results in the emission enhancement of the chromophore.[13]
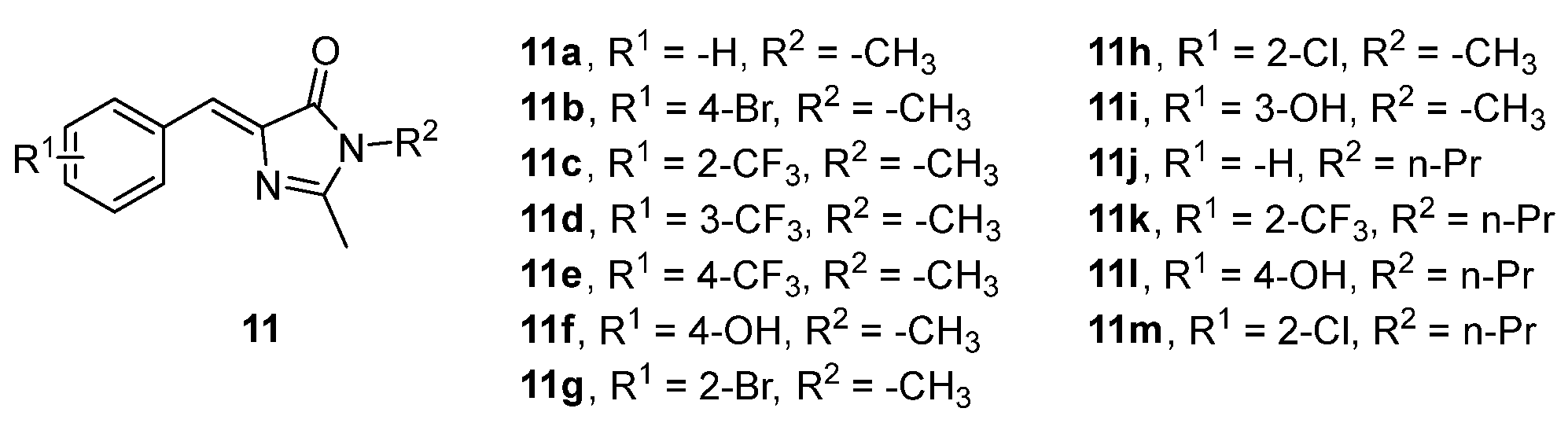
Figure 10. GFPc analogue with enhanced emission when encapsulated in NaCh vesicles.[13]
A β-cyclodextrin could mimic the protein environment, and act as a supramolecular host upon inclusion of a GFPc inside its cavity. The fluorescence intensity of a GFPc analogue (Figure 11) was clearly enhanced when it changed from the isolated chromophore 12 to the chromophore covalently attached to a β-cyclodextrin CD-12.[14] Here, the molecular motions of the GFPc, and the double bond isomerization, are blocked by its inclusion inside the cavity of the β-cyclodextrin.
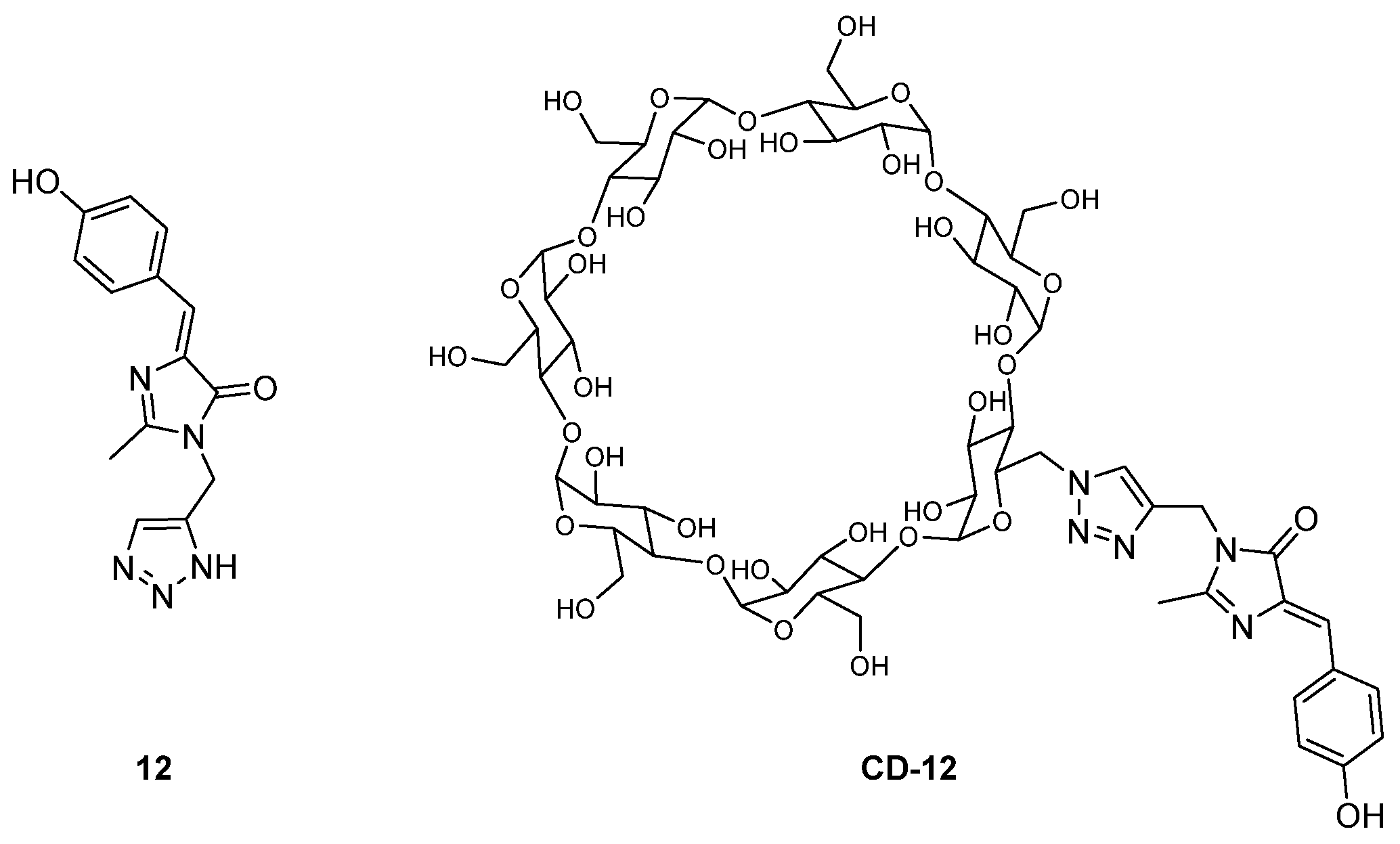
Figure 11. GFPc analogue isolated and attached to a β-cyclodextrin.[14]
A family of self-restricted GFPc analogues (chromophores 13a-c, Figure 12) has been designed to be self-restricting, based on previous report,[15] and their emission properties have been studied in solution, in the aggregate state and upon complexation inside a cyclodextrin cavity. In solution, the self-restricted chromophores 13b and 13c present higher emission when compared to chromophore 13a, due to the steric hindrance of the methoxy group, which limits the rotation of the benzylidene ring. GFPc 13c presents an enhanced emission compared to 13b probably due to the reduced hydrogen bonding effect.[16] The emission of 13a and 13b is quenched in the aggregate state, but 13c presents an enhanced emission intensity when it aggregates, which can be due to the segregation effect of the adamantyl group. Different β-cyclodextrins derivatives were used to study their effect on the fluorescence of these chromophores. Chromophore 13b does not seem to interact with the β-cyclodextrins, but GFPc 13c demonstrated an enhanced emission intensity on the presence of β-cyclodextrins, as the adamantyl group is known to readily enter the cyclodextrin cavity. The complexation with methyl-β-cyclodextrin proved to promote a higher emission.
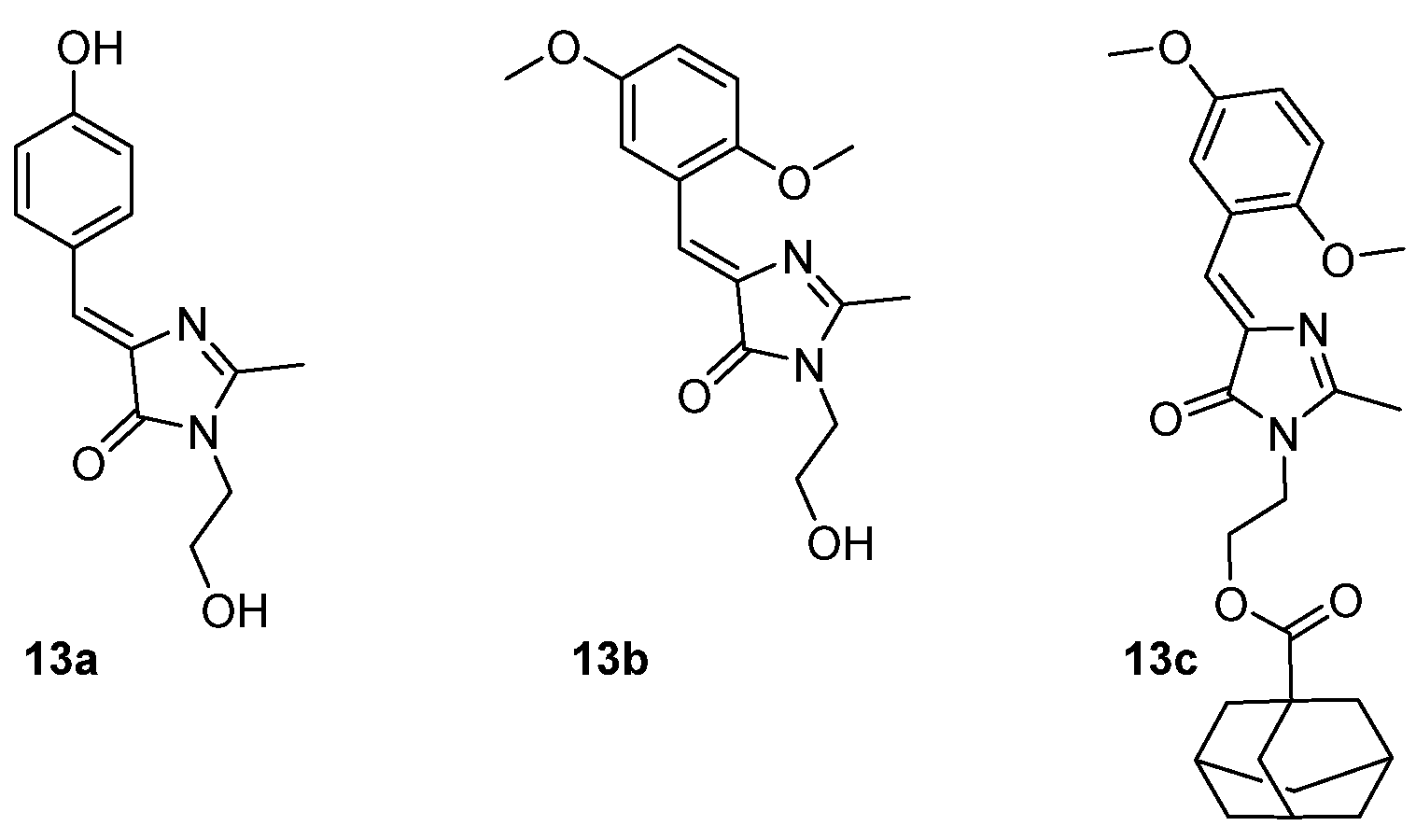
Figure 12. GFPc analogues used to study the supramolecular interaction with cyclodextrin.[16]
In a similar approach, the inner surface of the Tobacco Mosaic Virus (TMV) has been used to mimic the GFP β-barrel, as they are quite similar. Chromophore 14 (Figure 13) could be easily conjugated to the glutamate residues of the TMV channel through its amino group. After conjugation, the fluorescence intensity of TMV-14 exhibited a significant enhancement when compared to the isolated GFPc 14, with an extra enhancement in the presence of different organic solvents, with DMSO being the one that showed the most dramatic results.[17]
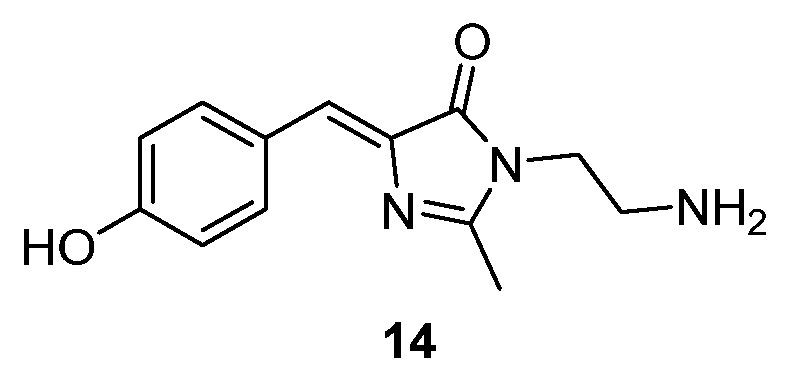
Figure 13. GFPc analogues that can be conjugated with the TMV channel.[17]
A GFPc has been modified in order to increase its affinity for β-amyloid fibrils and lysosomes (chromophore 15, Figure 14). The phenolic hydroxyl group of the GFPc has been replaced by a dimethylamino group, and a quinolone substituent has been introduced, in order to improve the affinity with β-amyloid fibrils. The 3-morpholinopropyl-amino group has been added as a lysosome-targeting group.[18] The GFPc 15 demonstrated to accumulate in the β-amyloids, which mimic the environment of the β-barrel of the GFP, thus turning on the fluorescence. The same effect was observed with lysosomes. Therefore, GFPc 15 allowed the detection of Aβ fibrils and the mapping of viscosity in lysosomes, with fluorescence emissions at 570 nm and 600 nm, respectively.
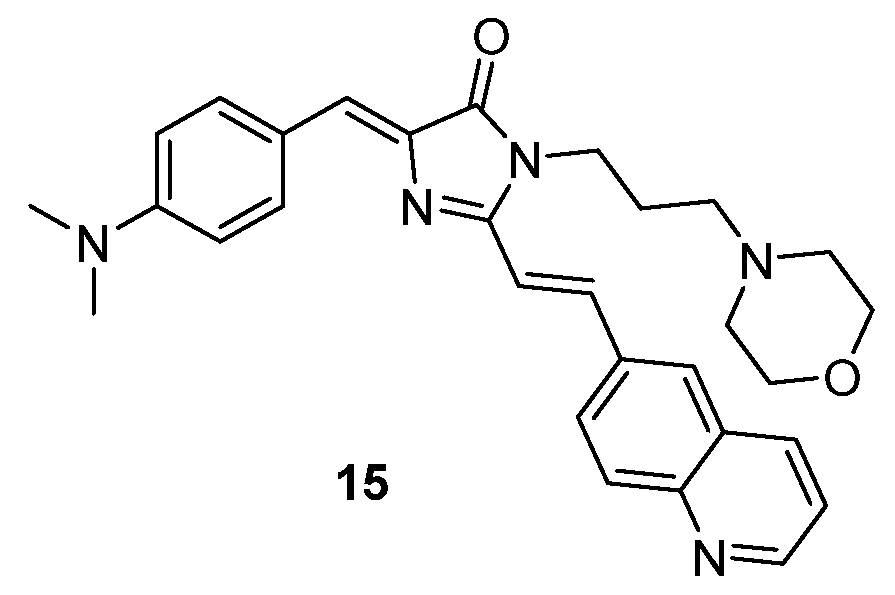
Figure 14. GFP analogues used as molecular probe for imaging β-amyloids and lysosomes.[18]
3. Polymers
The linking of GFP-inspired chromophores to a polymer chain has been proven effective in terms of enhancing the fluorescence emission. The polymer 16 (Figure 15) was designed by linking a GFPc analogue to the thermo-sensitive copolymer poly(ethylene glycol)-poly(N-isopropylacrylamide (PEG-PNIPAM) and exhibited a weak fluorescence at low temperature.[19] Copolymer 16 emits more brightly when above the lower critical solution temperature, which can be explained by the hindrance of the conformational motions of the GFPc due to the self-assembly of the PEG-PNIPAM blocks.
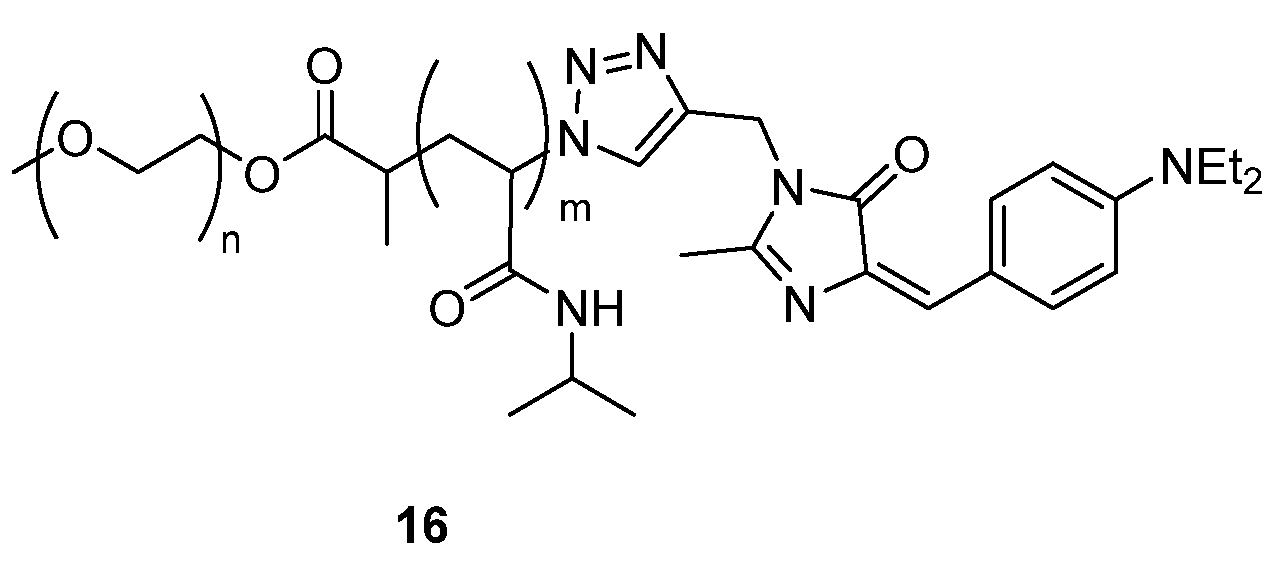
Figure 15. GFPc analogues linked to copolymer.[19]
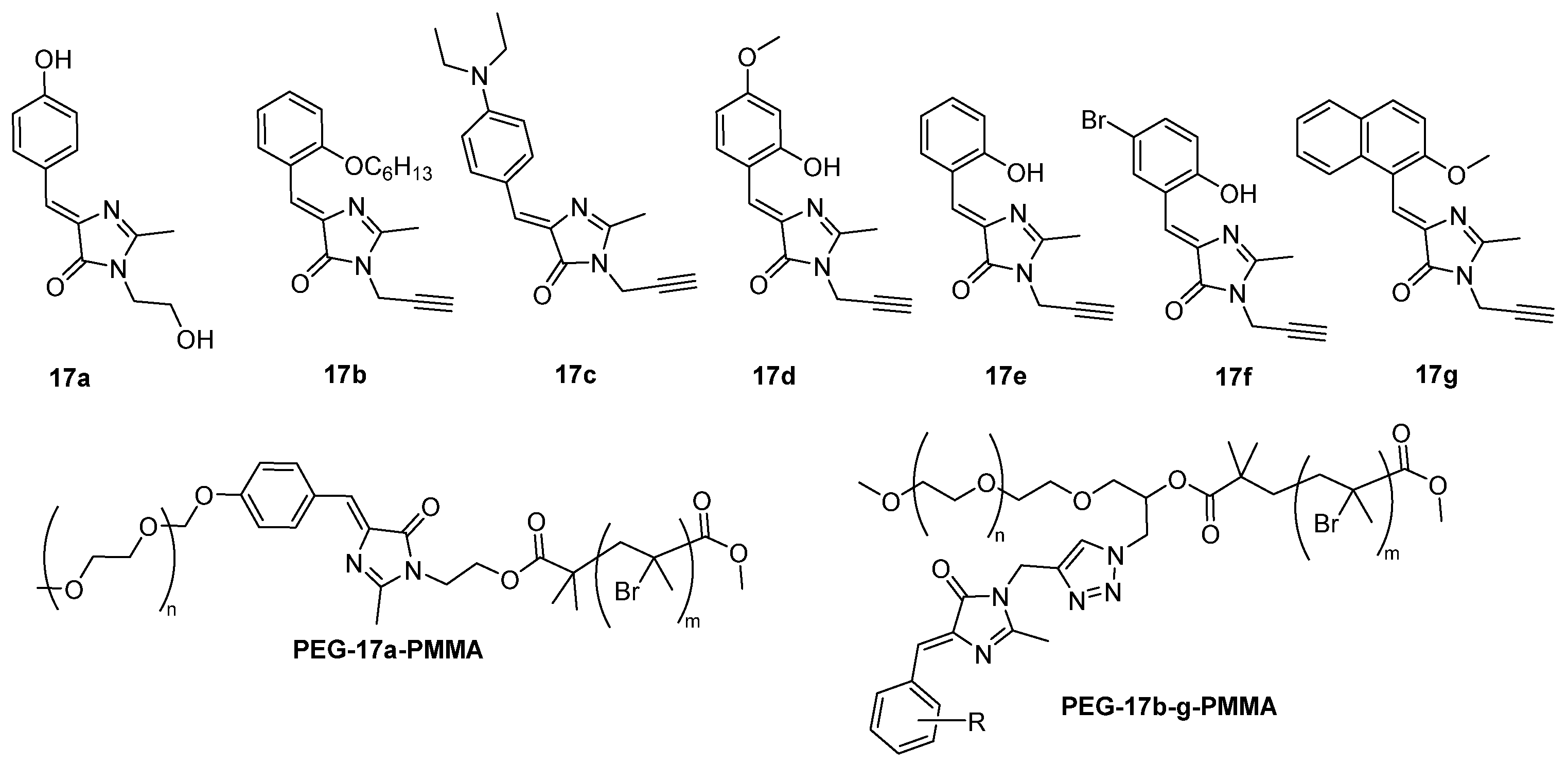
The copolymer can also be built around a single GFPc. For example, the copolymer poly(ethylene glycol)-17a-poly(methylmethacrylate) (PEG-17a-PMMA) has been grown on GFPc 17a (Figure 16). Compared to the isolated GFPc 17a, PEG-17a-PMMA demonstrated a red-shifted emission and an increased emission intensity (24 times) after the self-assembly of the GFP-copolymer into micellar aggregates.[20] This can be rationalized by the higher planarity of the chromophore and by the interactions between the chromophore and the copolymer. The GFP-copolymers poly(ethylene glycol)-17b-g-poly(methylmethacrylate) (PEG-17b-g-PMMA) include one chromophores 17b-g between the two parts of the copolymer (Figure 16). They also present a brighter fluorescence after assembly into micelles, with different emission wavelengths depending on the GFPc used (17b-g), varying between blue, green, yellow and orange.[21] This emission enhancement is related to the length of the hydrophobic chain, and is larger with longer chains, because these chains are able to reduce the intermolecular interactions and inhibit the molecular motions of the chromophore.
The connection of a GFPc with an organic spacer promotes the formation of a three-dimensional porous organic polymer with luminescent properties similar to the GFP. Two conjugated microporous polymers (18a-CMP and 18b-CMP, Figure 17) were prepared from two GFPc analogues (18a,b, Figure 17). The crystal structure of 18a revealed an intramolecular hydrogen bond between the proton of the hydroxyl group and the nitrogen of the imidazole. The quantum yields of the GFPc 18a and 18b are very low in methanol, mainly due to presence of the iodine atom which quenches the emission.[22] The molecular motions of the chromophores proved to be restricted in the three-dimensional network, and the emission properties of 18a-CMP are similar to the GFP.
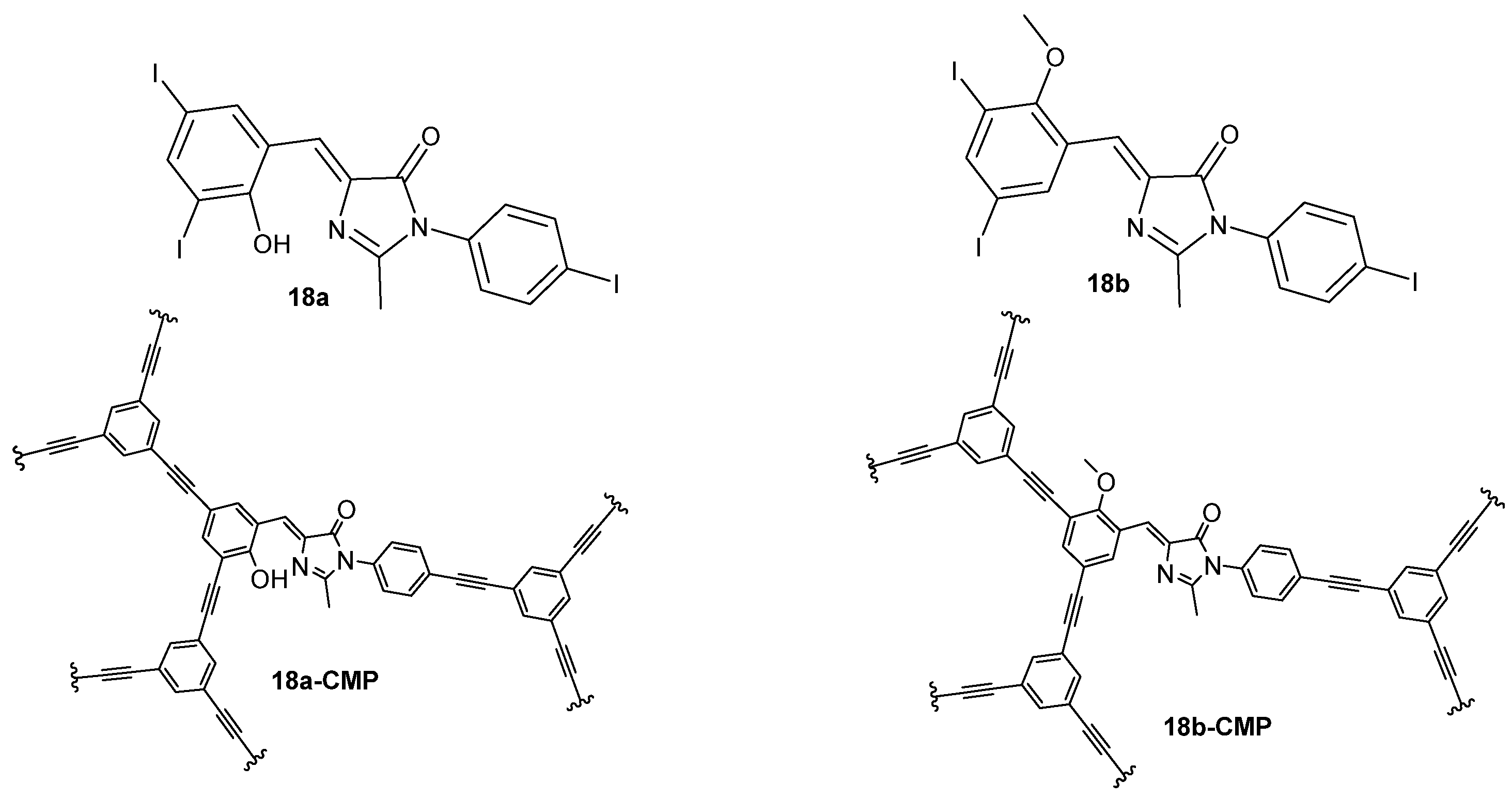
Figure 17. GFPc analogues used in the preparation of porous organic frameworks.[22]
The incorporation of a GFPc into a metal–organic framework was also reported as an alternative to mimic the behavior of the GFPc inside the β-barrel.[23] A metal–organic framework was prepared using GFPc 19 as linker (Figure 18) and zinc (II). It exhibits a green emission similar to the GFP, and the authors demonstrated that the molecular motions of the GFPc are hindered inside the framework.
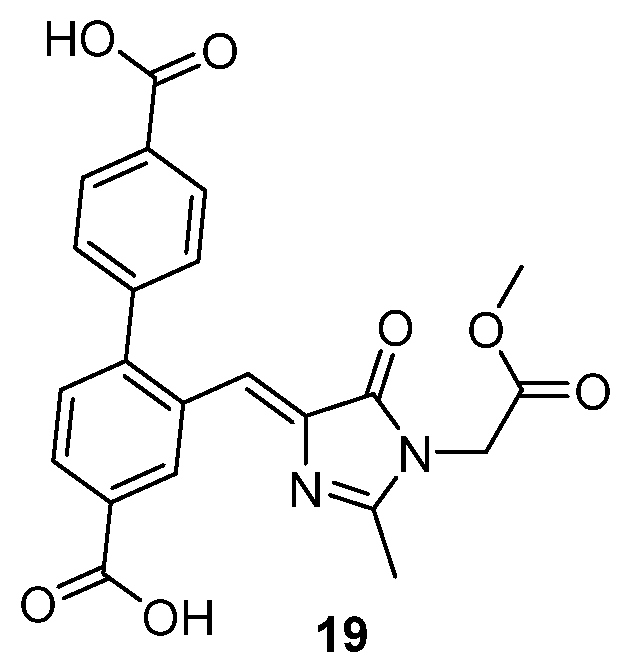
Figure 18. GFPc analogue used as linker in the preparation of a green-fluorescent metal–organic framework.[23]
References
- Esteves, C.I.C.; da Silva Fonseca, I.; Rocha, J.; Silva, A.M.S.; Guieu, S.; Synthesis and Luminescence Properties of Analogues of the Green Fluorescent Protein Chromophore.. Dyes and Pigments 2020, 177, 108267, 10.1016/j.dyepig.2020.108267.
- 11. Dong, J.; Solntsevn, K.M.; Tolbert, L.M.; Activation and Tuning of Green Fluorescent Protein Chromophore Emission by Alkyl Substituent-Mediated Crystal Packing.. J. Am. Chem. Soc. 2009, 131, 662, 10.1021/ja806962e.
- Shen, X.; Huang, G.; Li, K.; Zhang, G.; Zhang, D.; Tuning the Solid-State Emission of the Analogous GFP Chromophore by Varying Alkyl Chains in the Imidazolinone Ring. . Sci. China Chem. 2013, 56, 1197, 10.1007/s11426-013-4913-x.
- Ghodbane, A.; Brett Fellows, W.; Bright, J.R.; Ghosh, D.; Saffon, N.; Tolbert, L.M.; Fery-Forgues, S.; Solntsev, K.M.; Effects of the Benzoxazole Group on Green Fluorescent Protein Chromophore Crystal Structure and Solid State Photophysics.. J. Mater. Chem. C 2016, 4, 2793, 10.1039/c5tc03776j.
- Carayon, C.; Ghodbane, A.; Gibot, L.; Dumur, R.; Wang, J.; Saffon, N.; Rols, M.P.; Solntsev, K.M.; Fery-Forgues, S.; Conjugates of Benzoxazole and GFP Chromophore with Aggregation-Induced Enhanced Emission: Influence of the Chain Length on the Formation of Particles and on the Dye Uptake by Living Cells. . Small 2016, 12, 6602, 10.1002/smll.201602799.
- Carayon, C.; André-Barrès, C.; Leygue, N.; Saffon-Merceron, N.; Boggio-Pasqua, M.; Fery-Forgues, S.; The Role of the Synthetic Chromophore of GFP in Generating Polymorphism-Dependent on/off Photoluminescence.. Dyes and Pigments 2021, 187, 109119, 10.1016/j.dyepig.2020.109119.
- Mali, B.P.; Dash, S.R.; Nikam, S.B.; Puthuvakkal, A.; Vanka, K.; Manoj, K.; Gonnade, R.G.; Five Concomitant Polymorphs of a Green Fluorescent Protein Chromophore (GFPc) Analogue: Understanding Variations in Photoluminescence with π-Stacking Interactions. . Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2020, 76, 850, 10.1107/S2052520620010343.
- Huang, G.; Zhang, G.; Wu, Y.; Liao, Q.; Fu, H.; Zhang, D.; Modification of the Green Fluorescent Protein Chromophore with Large Aromatic Moieties: Photophysical Study and Solid-State Emission.. Asian J. Org. Chem. 2012, 1, 352, 10.1002/ajoc.201200086.
- Ikejiri, M.; Matsumoto, K.; Hasegawa, H.; Yamaguchi, D.; Tsuchino, M.; Chihara, Y.; Yamaguchi, T.; Mori, K.; Imanishi, T.; Obika, S.; et al.et al. Synthesis and Fluorescence Properties of 4-Diarylmethylene Analogues of the Green Fluorescent Protein Chromophore. . Tetrahedron 2015, 71, 4987, 10.1016/j.tet.2015.05.073.
- Baldridge, A.; Samanta, S.R.; Jayaraj, N.; Ramamurthy, V.; Tolbert, L.M.; Activation of Fluorescent Protein Chromophores by Encapsulation.. J. Am. Chem. Soc. 2010, 132, 1498, 10.1021/ja908870k.
- Lee, J.-S.; Baldridge, A.; Feng, S.; SiQiang, Y.; Kim, Y.K.; Tolbert, L.M.; Chang, Y.-T.; Fluorescence Response Profiling for Small Molecule Sensors Utilizing the Green Fluorescent Protein Chromophore and Its Derivatives. . ACS Comb. Sci. 2011, 13, 32, 10.1021/co100012k.
- Baldridge, A.; Feng, S.; Chang, Y.-T.; Tolbert, L.M.; Recapture of GFP Chromophore Fluorescence in a Protein Host. . ACS Comb. Sci. 2011, 13, 214, 10.1021/co200025e.
- Baldridge, A.; Amador, A.; Tolbert, L.M.; Fluorescence Turn On by Cholate Aggregates. . Langmuir 2011, 27, 3271, 10.1021/la2003244.
- Cacciarini, M.; Nielsen, M.B.; de Castro, E.M.; Marinescu, L.; Bols, M.; β-Cyclodextrin as a Mimetic of the Natural GFP-Chromophore Environment. . Tetrahedron Lett. 2012, 53, 973, 10.1016/j.tetlet.2011.12.05.
- Deng, H.; Yu, C.; Gong, L.; Zhu, X.; Self-Restricted Green Fluorescent Protein Chromophore Analogues: Dramatic Emission Enhancement and Remarkable Solvatofluorochromism. . J. Phys. Chem. Lett. 2016, 7, 2935, 10.1021/acs.jpclett.6b01251.
- Ge, S.; Deng, H.; Su, Y.; Zhu, X.; Emission Enhancement of GFP Chromophore in Aggregated State via Combination of Self-Restricted Effect and Supramolecular Host–Guest Complexation. . RSC Adv. 2017, 7, 17980, 10.1039/C7RA00974G.
- Zhou, Q.; Wu, F.; Wu, M.; Tian, Y.; Niu, Z.; Confined Chromophores in Tobacco Mosaic Virus to Mimic Green Fluorescent Protein.. Chem. Commun. 2015, 51, 15122, 10.1039/C5CC05751E.
- Sun, H.; Leng, H.X.; Liu, J. sheng; Roy, G.; Yan, J. wu; Zhang, L.; A Novel Fluorescent Protein Chromophore Analogue to Simultaneously Probe Lysosome Viscosity and β-Amyloid Fibrils.. Sens. Actuators B Chem. 2020, 305, 127509, 10.1016/j.snb.2019.127509.
- Zheng, Y.; Li, G.; Deng, H.; Su, Y.; Liu, J.; Zhu, X.; Temperature-Induced Fluorescence Enhancement of GFP Chromophore Containing Copolymers for Detection of Bacillus Thermophilus. . Polym. Chem. 2014, 5, 2521, 10.1039/c3py01559a.
- Deng, H.; Zhu, Q.; Wang, D.; Tu, C.; Zhu, B.; Zhu, X.; GFP-Inspired Fluorescent Polymer. . Polym. Chem. 2012, 3, 1975, 10.1039/c2py20223a.
- Deng, H.; Su, Y.; Hu, M.; Jin, X.; He, L.; Pang, Y.; Dong, R.; Zhu, X.; Multicolor Fluorescent Polymers Inspired from Green Fluorescent Protein. . Macromolecules 2015, 48, 5969, 10.1021/acs.macromol.5b01166.
- Singh, A.; Samanta, D.; Boro, M.; Maji, T.K.; Gfp Chromophore Integrated Conjugated Microporous Polymers: Topological and ESPT Effects on Emission Properties. . Chem. Commun. 2019, 55, 2837, 10.1039/C9CC00357F.
- Williams, D.E.; Dolgopolova, E.A.; Pellechia, P.J.; Palukoshka, A.; Wilson, T.J.; Tan, R.; Maier, J.M.; Greytak, A.B.; Smith, M.D.; Krause, J.A.; et al.et al. Mimic of the Green Fluorescent Protein β-Barrel: Photophysics and Dynamics of Confined Chromophores Defined by a Rigid Porous Scaffold. . J. Am. Chem. Soc. 2015, 137, 2223, 10.1021/ja5131269.
More