You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Hesham El-Seedi.
The kingdom of Saudi Arabia (SA) ranks fifth in Asia in terms of area. It features broad biodiversity, including interesting flora, and was the historical origin of Islam. It is endowed with a large variety of plants, including many herbs, shrubs, and trees. Many of these plants have a long history of use in traditional medicine.
The kingdom of Saudi Arabia (SA) ranks fifth in Asia in terms of area. It features broad biodiversity, including interesting flora, and was the historical origin of Islam. It is endowed with a large variety of plants, including many herbs, shrubs, and trees. Many of these plants have a long history of use in traditional medicine.
- Saudi Arabian plants
- distribution
- anti-inflammation
- anti-cancer
1. Introduction
The plants of Saudi Arabia (SA) have high biological diversity in the Arabian Peninsula, where they represent an important genetic resource for crop and medicinal plants. About 30% of these plants are rare due to the geographical location and dry weather characteristics of SA [1,2][1][2]. Various parts of the plants are used in traditional medicine, including the bark, flowers, fruits, leaves, resins, rhizomes, roots, seeds, and stems. All plants contain primary metabolites, while secondary metabolites serve a specific plant species in the interaction with its environment. The contents of biologically active compounds are plant-specific and genetically determined; however, they are also affected by the cultivation, practices, pests and diseases, climate, weather, stage of development (season, young and old leaves), ecosystem, and the time of the day the material is collected [3]. Because of the extreme conditions in SA, plants have developed different strategies to survive. In terms of phytochemistry, this results in high levels of secondary metabolites, such as flavonoids, polyphenols, terpenes, tannins, saponins, sterols, alkaloids, and their glycosides [3].
The importance of natural products for novel drug development is obvious from present-day pharmacology, and many natural products have served as the basis for developing medicines and are still used as medicines for various ailments. Morphine and codeine from Papaver somniferum L., quinidine, and quinine from Cinchona spp., atropine from Atropa belladonna L., and digoxin from Digitalis spp. are prominent examples of well-established plant-derived pharmaceutical preparations [4]. Newman and Cragg [5] demonstrated that almost one-half of all novel medicines developed in recent decades are natural products or natural product derivatives and analogues. Therefore, searching nature for novel hits for the development of medicine is increasing [6].
Novel medicines are required because of several problems with present-day pharmaceuticals, including severe side effects and resistance to anti-cancer medicines or antibiotics. For example, the common non-steroidal anti-inflammatory drugs (NSAIDs) are known for their side effects, such as cardiovascular incidents and gastrointestinal bleeding [7]. A recent study conducted in SA reported that the side effects of NSAIDs and antibiotics were reported by 80.7% (n = 272) and 48.7% (n = 164) of users, respectively [8]. Novel NSAIDs with fewer side effects are needed. In addition to the side effects of antibiotics, their uncontrolled use increases the likelihood of microorganisms developing resistance, and thus, increases the number of severe infections and mortality [9]. Cancer incidence in SA has increased in the past 27 years; thyroid cancer incidence increased by 26-fold and breast, bladder, colon, and uterine cancer incidences increased by about 10-fold. The number of cancer deaths in SA increased from 5% in 1990 to 12% in 2016 [10].
Saudi citizens are dependent on both traditional medicinal plants and modern medicines, as was reported in a review on the ethnopharmacology of SA plants [11]. Although several publications report on traditional medicines in SA [12[12][13][14],13,14], there is no comprehensive review about the total plant biodiversity in SA and its uses to date. Combining traditional knowledge with modern pharmacognostic research, novel hits for drug development may be found, resulting in the evidence-based use of traditional medicines. Other recent studies have shown interesting non-medical applications of secondary metabolites from Saudi Arabian plants. An example of a plant from SA with a high content of valuable compound(s) is Plectranthus aegyptiacus, a source of thymol, which is the main component of the plant’s essential oil (58.49%). Thymol is a volatile phenolic monoterpenoid; among other uses, it is used in food conservation [15,16,17,18][15][16][17][18].
2. Distribution and Diversity of the Flora of Saudi Arabia
SA is the largest country in the Arabian Peninsula, occupying about 2.25 million km2, or nearly 80% of the peninsula [21][19]. Forests cover about 27,000 km2 of its total area, mostly in the southwestern part of the Kingdom [22][20], while the area of natural rangeland exceeds 1.75 million km2 [23][21]. Plant diversity plays a key role in maintaining and preserving ecological balance and stability, which is an important factor for human well-being, not only for the country but for the whole world [24][22]. In recent years, a significant amount of data have been published about the flora in SA, which revealed that the country is gifted with a wide variety of plants, including a large number of herbs, shrubs, and trees that are found in desert, semi-desert, and mountainous ecosystems [25,26,27][23][24][25]. Geographic and climatic diversity, changes in water resources, drought, and anthropogenic pressures, such as overgrazing, deforestation, and all developmental activities in SA, negatively affect biodiversity, impacting the structure of the flora and natural vegetation [22,28][20][26]. The climate of SA varies greatly depending on the area and the season; it is characterised by extremes in weather, with very high temperatures during the daytime followed by an abrupt drop in temperature at night and very little, irregular precipitation (i.e., a typical ‘desert climate’) [21][19]. The annual mean temperature of the country is 24.64 °C, with Turaif in the north recording the lowest temperature (19.10 °C) and Makkah in the west recording the highest temperature (31.69 °C), with an average of 17.62 °C, exhibiting an increasing gradient from north to south [29][27]. Rainfall and temperature are considered the key controllers of agricultural production [30][28]. The annual average rainfall in SA from 1979 to 2009 was 93.5 mm, and it is expected that by 2050, some parts of SA will experience a decrease in rainfall by 20–25% in dry seasons and 10–15% in the winter. The maximum temperature has increased significantly, especially in dry seasons, by 0.72 °C per decade [31][29]. SA is home to about 2247 plant species, including native and introduced plants that belong to 142 families and 837 genera, and the components of its flora are the admixture of the features of Africa, Asia, and the Mediterranean region in addition to a large number of about 246 endemic species [32,33,34,35,36][30][31][32][33][34]. Of them, about 105 species reside in dunes, 90 species are halophytes, 75 species are trees, and aquatic plants comprise 12 species. About 450 species of total flora have a direct benefit to humans; 334 species have medicinal value, 38 species are palatable fodder, 25 species are human food, 45 species are poisonous, 47 species are used as ornamentals, and 471 species are used in ethnomedicine [22,37][20][35]. Of note, about 600 species of plants in SA are rare and endangered in their habitat [38][36]. Mecca city district is characterised by various types of plants; it was reported that a total number of 184 species of 125 genera and 44 families are found in Mecca. The most well-known families are Poaceae (17%), Fabaceae (13%), and Amaranthaceae (5%) [32][30]. Moreover, wadis represent one of the most notable desert landforms where physiographic irregularities appear, leading to parallel variation in species distribution and life forms. In wadi Al-Noman in Mecca, about 126 species from 39 families have been recorded, namely Fabaceae, Poaceae, and Boraginaceae. The authors indicated that species diversity in the studied area may be positively associated with the high pH and mineral content of the soil [39][37]. The vegetation along a transect between Makkah Al-Mukarramah and Al-Madinah Al-Munawarah in the Hejaz Mountains includes 106 species of vascular plants from 35 families [40][38]. In a survey of a sector in the Hejaz mountains along the Medina-Badr road, 247 species of 173 genera and 52 families are listed [41][39]. In Jizan province in the southern region, with varied landscapes such as wadis, islands, plateaus, and mountains, 850 species of 434 genera from 98 families represent about 37% of all the species in SA. Of them, 536 species are from Jabal Fayfa and 202 species are from the Farasan Islands. The most dominant families are Poaceae, Papilionaceae, and Asteraceae, with a total of 87, 70, and 60 species, respectively [42][40]. In a survey of the flora of the Sarwat Mountains in Taif as showed in Figure 1, 261 species from 178 genera and 55 families were recorded, of which Asteraceae, Poaceae, Fabaceae, Lamiaceae, Chenopodiaceae, Boraginaceae, Asclepiadaceae, Brassicaceae, and Zygophyllaceae were the most common [43][41]. In another mountainous area, the Asir mountains in the southwest of SA, 189 species of 74 families were recorded; Asteraceae was the dominating family in the study area (36.5%) followed by hemicryptophytes (15%) and geophytes (12.5%) [28][26]. In the eastern part of the Kingdom, no more than 370 native species of herbs and shrubs are adapted to the desert climate, reflecting the difficulties faced by plants in these harsh environments; the same applies for the northern part [44][42]. This is in contrast with Asir Hijaz, the western mountainous area of the Kingdom, which has large species diversity due to the greater rainfall [33][31]. The number of recorded species is still increasing through new biodiversity surveys [32][30]; however, the richness and diversity of the flora might be lost due to anthropogenic pressure and changes in climate [37,45][35][43]. Therefore, further studies are needed to ecologically and taxonomically evaluate all the flora of the country and to provide suitable conservation measures for these valuable species with unique features.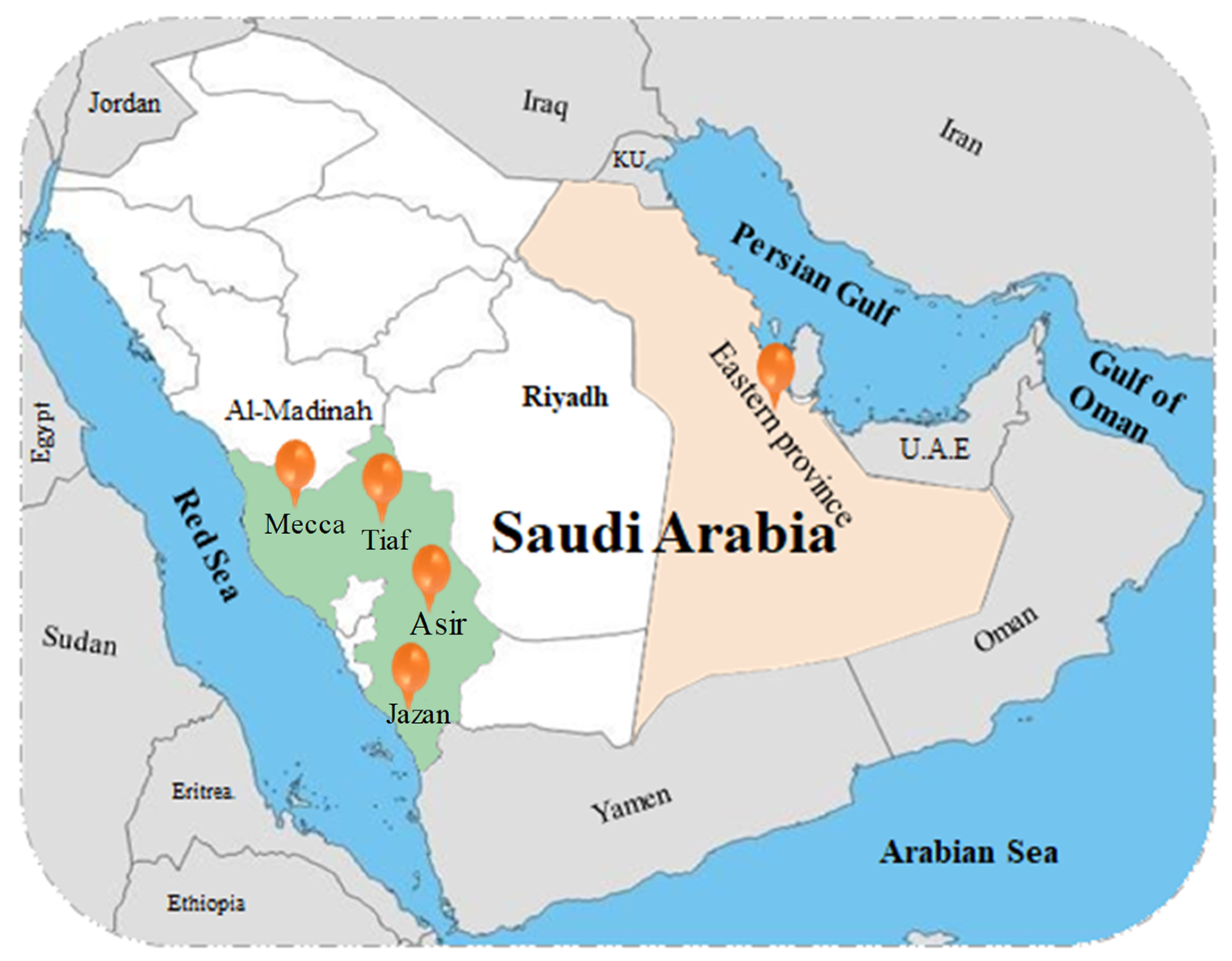
Figure 1. A map showing the surveys sites of different ecological areas in Saudi Arabia (SA).
3. History of Medicinal Plants in Saudi Arabia
People have used plants to meet their various needs throughout different eras. Plants have formed the basis of traditional medicine for at least 6000 years in Mesopotamia and Egypt, with Hippocrates serving as a landmark for the written traditions on medicines [46,47][44][45]. Traditional medicine has been and is still a part of the culture of SA [48][46]. SA has some unique features besides its religious position. Zamzam Well is one of the most well-known places in SA, known for its suitable environment for many herbs and shrubs, as mentioned in the verses of the Qur’ân: Our lord, I have settled some of my offspring in a barren valley, near your sacred House, our Lord, that they may establish prayer. So make the hearts of people incline towards them and provide them with sustenance that they may give thanks (Verse # 37, Surah Ibrahim, p. 260). Agriculture has been the main source of economic income for several Saudi regions, even during the pre-Islamic times, and farmers followed both advanced and traditional irrigation methods by diverting rainwater flowing into agricultural terraces or bringing rainwater drawn from dams to irrigate parched wadis [43][41]. From the 7th century, SA has been the historical origin of Islam. ‘Traditional Islamic medicine’ is the term used to describe medical traditions thriving during Islam’s golden age [49][47]. In fact, many plants are highly recommended by Prophet Muhammad (peace be upon him (PBUH)). Other plants are mentioned in different surahs of the Holy Qur’ân, such as date palm, olive, toothbrush tree, camphor tree, squash, ginger, onion, grapes, clover [50][48]. Prophet Muhammad (PBUH) mentioned the use of Miswak (Salvadora persica L.) for oral hygiene [51][49]. The Prophet (PBUH) said, ‘Siwak (Miswak) is a means of purification for the mouth and is pleasing to the Lord’ [52][50]. At that time, the use of medicinal plants to prevent and treat health disorders flourished, and philosophers and physicians, such as Al-Razi and Ibn Sina, began to change the concept of medicine. They compiled medical books from the knowledge available in the Mediterranean region (e.g., from Egyptian, Persian, Greek, and Roman times) that have been used for centuries (700–1500 AD). This knowledge was selected through controlled experimentation and sound reasoning. ‘The Canon of Medicine’, ‘The Book of Healing’, and ‘Kitab Al-Hawi’ are the most famous books written by Ibn Sina (Father of Early Modern Medicine) and Al-Razi [50,53][48][51]. For these reasons, people in SA have taken an interest in plants; about 80% of Saudi citizens have used medicinal plants for their primary health care [11]. Recent publications reported the activity of S. persica (Miswak, toothbrush tree) against oral bacteria that cause caries. This species showed effective antimicrobial, antiulcer, analgesic, anti-inflammatory, and antitumour activities, which agrees with the recommendations of the Prophet (PBUH) [54][52]. The uses of 12 medicinal plants that were recommended by the Prophet (PBUH) and mentioned in Qur’ânic verses were recently published [50,55][48][53].4. Anti-Inflammatory Activity of Saudi Arabian Plants
People have always been exposed to inflammatory diseases; the clinical symptoms were first described by the Roman physician Cornelius Celsus in the first century A.D. [56][54]. Inflammation is an immune response to various external factors, such as infection with microorganisms, toxic compounds, damaged cells, or irradiation [57][55]. An inflammatory response is a multi-step process initiated by inducers that are detected by specific receptors (sensors) on specialised sentinel cells (e.g., mast cells, macrophages, and dendritic cells). Sensors stimulate the production of mediators, such as cytokines, chemokines, eicosanoids, bioactive amines, and products of proteolytic cascades, which affect target tissues [56][54]. Such inflammatory inducers may lead to acute and/or chronic inflammatory responses in various body organs (e.g., liver, heart, brain, lung, intestinal tract, kidney, pancreas, and reproductive system), resulting in tissue damage or disease [58,59][56][57]. Worldwide, three out of five people die due to chronic inflammation caused by cardiovascular diseases, cancer, stroke, bowel diseases, chronic respiratory diseases, arthritis, obesity, and diabetes [60][58]. Unfortunately, the available anti-inflammatory drugs are associated with a number of side effects, including cardiovascular effects and gastrointestinal bleeding [7]. Therefore, there is a need for new potent drugs with fewer side effects for better management of inflammatory ailments. Natural products, especially medicinal plants, are a rich source of phytochemicals, which have been shown to relieve pain or have anti-inflammatory effects [61,62,63][59][60][61]. For example, curcumin isolated from Curcuma longa L. was the subject of several clinical studies of inflammatory diseases [64][62]. In-depth studies are needed to identify the mode of action and to optimise the pharmacological profile of plant extracts and active compounds. Regarding the native plants of SA, several have been found to inhibit either the production of pro-inflammatory mediators or decrease their action, leading to anti-inflammatory activity [65][63]. Essential oils from Achillea fragrantissima Sch. Bip. and Lactuca serriola L., two plants of the family Asteraceae that grow in SA, were investigated for their anti-inflammatory activity; they were shown to exhibit a high inhibition of carrageenan-induced oedema after 4 h at a dose of 100 mg/kg orally by 71.9% and 73.4%, respectively [66][64]. The promising effects were due to the presence of azulene and oxazolidine in both A. fragrantissima and L. serriola [66][64]. Elsharkawy et al. [67][65] examined the anti-inflammatory effects of a mixture of three different plants (Artemisia herba alba, Rubia tinctorum, and Alkanna tinctoria) used by Bedouins in SA to treat many skin diseases. The oil mixture of these plants showed anti-inflammatory activity with oedema inhibition after 4 h (78.0% with 100 mg/kg orally) compared to the standard drug (89.5% with 10 mg/kg indomethacin) [67][65]. Using a bioassay-guided approach to identify the anti-inflammatory activity of compounds in Cadaba glandulosa Forssk, four active compounds were isolated and identified. The paw oedema thickness of rats was found to be 3.04, 3.11, 4.16, and 4.89 mm 4 h after the application of compounds 3, 2, 1, and 4, respectively, at 10 mg/kg subcutaneously compared with the standard drug (indomethacin 10 mg/kg reduced oedema thickness to 4.47 mm) [68][66]. Regarding the structure–activity relationship (SAR), compound 3 exhibited the highest anti-inflammatory activity in this study. The total MeOH extract (100 mg/kg) of C. glandulosa Forssk reduced the oedema thickness to 4.29 mm after 4 h [68][66]. Six triterpenes (compounds 5–10) of Kleinia odora were evaluated for their anti-inflammatory activity, causing a decrease in pro-inflammatory genes like nuclear transcription factor-kB (NF-κB) and cascaded interleukins (IL-6, IL-1β, and TNF-α) [69][67]. Only compounds 5 and 6 produced a notable change, decreasing oedema thickness at a low dose of 0.4 μg/mL, but other compounds (7–10) showed only good anti-inflammatory activity at 40 μg/mL. Regarding skin SAR, the activity may be due to the lupane nucleus with ketone or acetate at position C-3, as in compounds 6 and 5 in Figure 2, respectively; however, the presence of an OH- group at position 3 causes a decrease in activity, as for compounds 8 and 9 [69][67]. Hexane and chloroform extracts of Huernia Sp. Nov. aff. Boleana exhibited good activity, as the rats’ tail volume decreased and wound repair and re-epithelisation were enhanced [70][68]. Additional plants and active compounds with potent anti-inflammatory activity from the central and western regions of SA have been reported (Table 1).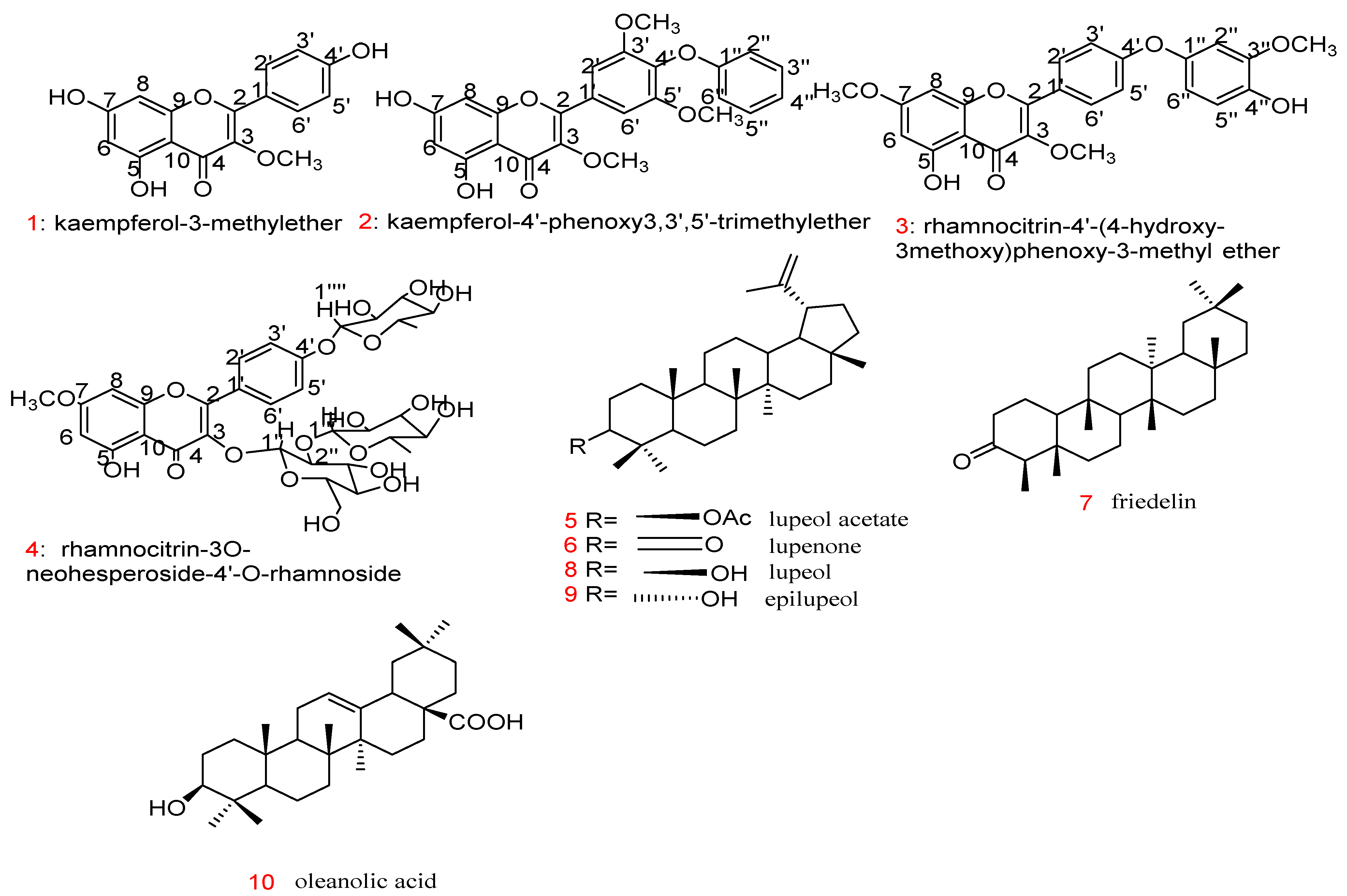
Figure 2. Structure of compounds 1 to 10 with anti-inflammatory effects.
Table 1. The biological activities of isolated compounds from plants in the central and western regions of SA.
| S. No. | Plant/Family | Location/Time | Solvent/Part Used | Animal, Cell Line, Organism, or Method Used | Activity | Ref. | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 23 | Premna resinosa | (Hochst.) Schauer/Lamiaceae | Riyadh (central)/Jan. 2011 region | Methanol extract/aerial parts | Daoy, HepG2, and SK-MEL28/DPPH Scavenging activity (DPPH SCA) assay | Cytotoxic activity just against Daoy cell line with IC | 50 | = 10.5 μg/mL PC = 7.3 μg/mL Antioxidant activity via inhibition of the discoloration of β-carotene by 94.4% at 1000 μg/mL PC = 93.1% |
[71] | [69] | |||||||||||||||
| Commiphora opobalsamum | L./Burseraceae | Near Abha city (Southern)/2003 | Various fractions/aerial parts | DCFH-DA method/HL-60 cells | Antioxidant activity with IC | 50 | = 0.55 μg/mL PC = 0.39 μg/mL |
[72] | [70] | ||||||||||||||||
| 24 | P. resinosa | (Hochst.) Schauer/Lamiaceae | Riyadh (central)/Jan. 2011 | Methanol extract/aerial parts | HepG2, Daoy, and SK-MEL28/DPPH SCA assay | Cytotoxic activity just against Daoy cell line with IC | 50 | = 21.1 μg/mL PC = 7.3 μg/mL Antioxidant activity via inhibition of the discoloration of β-carotene by 91.2% at 1000 μg/mL PC = 93.1% |
[71] | [69] | |||||||||||||||
| 25 | P. resinosa | (Hochst.) Schauer/Lamiaceae | Riyadh (central)/Jan. 2011 | Methanol extract/aerial parts | HepG2, Daoy, and SK-MEL28/DPPH SCA assay | Cytotoxic activity just against Daoy cell line with IC | 50 | = 7 μg/mL PC = 7.3 μg/mL Antioxidant activity via inhibition of the discoloration of β-carotene by 80.9% at 1000 μg/mL PC = 93.1% |
[71] | [69] | |||||||||||||||
| 26 | P. resinosa | (Hochst.) Schauer/Lamiaceae | Riyadh (central)/Jan. 2011 | Methanol extract/aerial parts | Daoy, HepG2, and SK-MEL28/DPPH SCA assay | Cytotoxic activity just against Daoy cell line with IC | 50 | = 19.6 μg/mL PC = 7.3 μg/mL Antioxidant activity via inhibition of the discoloration of β-carotene by 90.5% at 1000 μg/mL PC = 93.1% |
[71] | [69] | |||||||||||||||
| 1 | P. resinosa | (Hochst.) Schauer/Lamiaceae | Riyadh (central)/Jan. 2011 | Methanol extract/aerial parts | Daoy, HepG2, and SK-MEL28/DPPH SCA assay | Cytotoxic activity just against SK-MEL28 cell line with IC | 50 | = 18.8 μg/mL PC = 23.8 μg/mL Antioxidant activity via inhibition of the discoloration of β-carotene by 86.2% at 1000 μg/mL PC = 93.1% |
[71] | [69] | |||||||||||||||
| 38 | , | 39 | , | 40 | , | 41 | , | 42 | , | 43 | , | 44 | , | 45 | , | 24 | , and | 46 | Sisymbrium irio L/Cruciferae | Najed Region (central)/Apr. 2008 | Ethanol extract/aerial parts | Scavenging of the ABTS radical | Antioxidant activity ranged from 372 to 785 μM Trolox equivalent/g dry weight | [73] | [71] |
| 52 | , | 53 | , | 54 | , | 47 | , | 55 | , | 56 | , | 35 | , | 57 | , and | 58 | Cordia sinensis | /Boraginaceae | Riyadh (Central) | Methanol extract/dried plant | Carrageen-induced paw oedema of rats | Anti-inflammation-inhibited oedema ranged from 38.4 to 62.4% PC = 57.6% Anti-glycation activity ranged from 68 to 88.4% PC = 86% |
[74] | [72] | |
| 68 | Sarcocornia fruticose | /Chenopodiaceae | Al-Kharrar lagoon-Red Sea, Jeddah (west)/Apr. 2016 | Methanol extract/leaves | HCV Protease inhibitory assay | Showed inhibition of HCV protease with IC | 50 | = 8.9 μM PC = 1.5 μM |
[75] | [73] | |||||||||||||||
| 69 | and | 70 | S. fruticose | /Chenopodiaceae | Al-Kharrar lagoon-Red Sea, Jeddah (west)/Apr. 2016 | Methanol extract/leaves | DPPH SCA assay | Antioxidant activity with IC | 50 | = 3.8 and 4.3 μM of compounds 69 and 70, respectively PC = 1.5 μM |
[75] | [73] | |||||||||||||
| 71 | P. punctulata | /Asteraceae | Al-Kharrar lagoon-Red Sea, Jeddah (west)/Apr. 2016 | Methanol extract/root | Jurkat and HeLa cell lines/ | The antiproliferative activity caused growth inhibition with IC | 50 | = 12 and 18 µM of Jurkat and HeLa cells, respectively, due to inducing cell cycle arrest in G0/G1. Lowering the basal level of peroxides DHFDA-load in the cell. |
[75] | [73] | |||||||||||||||
| 73 | , | 74 | , | 75 | , | 23 | , and | 60 | Cassia italica | /Fabaceae | Gabal Al-Ateeq, Al Madinah Al Munawwarah (west)/Apr. 2017 | Ethyl acetate fraction/aerial parts | DPPH assay | Potent antioxidant activity ranges from 19.7 to 95.8%, compared to butylated hydroxyanisole 93.8% |
[76] | [74] | |||||||||
| 37 | Psiadia punctulata/ | Asteraceae | Wadi Ghazal (west)/Jun. 2012 | Dichloromethane surface extract/leaves | Biofilm inhibition against | C. Albicans | and | S. aureus | Reduce the biofilm formation of both | S. aureus | and | C. Albicans | at 40 μg/mL concentration by 50% and 90%, respectively | [77] | [75] |
References
- Osman, A.K.E.; Abdein, M.A.E.-H. Floristic diversity of Wadi Ar’ar, Saudi Arabia. J. Taibah Univ. Sci. 2019, 13, 772–789.
- Abdelmohimen, M.A.H.; Algarni, S.A. Numerical investigation of solar chimney power plants performance for Saudi Arabia weather conditions. Sustain. Cities Soc. 2018, 38, 1–8.
- Alqethami, A.; Aldhebiani, A.Y. Medicinal plants used in Jeddah, Saudi Arabia: Phytochemical screening. Saudi J. Biol. Sci. 2021, 28, 805–812.
- Rates, S.M.K. Plants as source of drugs. Toxicon 2001, 39, 603–613.
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803.
- El-Seedi, H.R.; Burman, R.; Mansour, A.; Turki, Z.; Boulos, L.; Gullbo, J.; Göransson, U. The traditional medical uses and cytotoxic activities of sixty-one Egyptian plants: Discovery of an active cardiac glycoside from Urginea maritima. J. Ethnopharmacol. 2013, 145, 746–757.
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018, 9, 143.
- Wajid, S.; Siddiqui, N.A.; Mothana, R.A.; Samreen, S. Prevalence and Practice of Unused and Expired Medicine—A Community-Based Study among Saudi Adults in Riyadh, Saudi Arabia. BioMed Res. Int. 2020, 2020, 6539251.
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241.
- Althubiti, M.A.; Eldein, M.M.N. Trends in the incidence and mortality of cancer in Saudi Arabia. Saudi Med. J. 2018, 39, 1259.
- Ullah, R.; Alqahtani, A.S.; Noman, O.M.A.; Alqahtani, A.M.; Ibenmoussa, S.; Bourhia, M. A review on ethno-medicinal plants used in traditional medicine in the Kingdom of Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 2706–2718.
- Ai-Said, M.S. Traditional medicinal plants of Saudi Arabia. Am. J. Chin. Med. 1993, 21, 291–298.
- Orfali, R.; Perveen, S.; Siddiqui, N.A.; Alam, P.; Alhowiriny, T.A.; Al-Taweel, A.M.; Al-Yahya, S.; Ameen, F.; Majrashi, N.; Alluhayb, K. Pharmacological evaluation of secondary metabolites and their simultaneous determination in the Arabian medicinal plant Plicosepalus curviflorus using HPTLC validated method. J. Anal. Methods Chem. 2019, 2019, 7435909.
- Khan, M.; Khan, M.; Abdullah, M.M.S.; Al-Wahaibi, L.H.; Alkhathlan, H.Z. Characterization of secondary metabolites of leaf and stem essential oils of Achillea fragrantissima from central region of Saudi Arabia. Arab. J. Chem. 2020, 13, 5254–5261.
- Shaheen, U.; Khalik, K.A.; Abdelhady, M.I.S.; Howladar, S.; Alarjah, M.; Abourehab, M.A.S. HPLC profile of phenolic constituents, essential oil analysis and antioxidant activity of six Plectranthus species growing in Saudi Arabia. J. Chem. Pharm. Res. 2017, 9, 345–354.
- Escobar, A.; Perez, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269.
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Elnesr, S.S. A review on the beneficial effect of thymol on health and production of fish. Rev. Aquac. 2021, 13, 632–641.
- Daradka, H.M.; Aljohani, H.A.; Alotaibi, M.K.; Khabour, O.F.; Eskandrani, A.A.; Alsharif, S.M.; Al-shdefat, R.; Abu-Harirah, H.A.; Bataineh, Y. Evaluating the effects of Commiphora molmol (myrrh) against oxidative and damage in human lymphocytes. Int. J. Pharm. Sci. Amp. 2021, 12, 3143–3149.
- Hasanean, H.; Almazroui, M. Rainfall: Features and variations over Saudi Arabia, a review. Climate 2015, 3, 578–626.
- Aati, H.; El-Gamal, A.; Shaheen, H.; Kayser, O. Traditional use of ethnomedicinal native plants in the Kingdom of Saudi Arabia. J. Ethnobiol. Ethnomed. 2019, 15, 2.
- Daur, I. Plant flora in the rangeland of western Saudi Arabia. Pak. J. Bot 2012, 44, 23–26.
- Haines-Young, R.; Potschin, M. The links between biodiversity, ecosystem services and human well-being. Ecosyst. Ecol. N. Synth. 2010, 1, 110–139.
- Masrahi, Y.; Al-Huqail, A.; Al-Turki, T.; Thomas, J. Odyssea mucronata, Sesbania sericea, and Sesamum alatum–new discoveries for the flora of Saudi Arabia. Turk. J. Bot. 2012, 36, 39–48.
- Ali, N.A.A.; Al Sokari, S.S.; Gushash, A.; Anwar, S.; Al-Karani, K.; Al-Khulaidi, A. Ethnopharmacological survey of medicinal plants in Albaha Region, Saudi Arabia. Pharmacogn. Res. 2017, 9, 401.
- Thomas, J.; Sivadasan, M.; Al-Ansari, A.M.; Alfarhan, A.; El-Sheikh, M.; Basahi, M.; Alatar, A.A. New generic and species records for the flora of Saudi Arabia. Saudi J. Biol. Sci. 2014, 21, 457–464.
- Al-Yemeni, M.; Sher, H. Biological spectrum with some other ecological attributes of the flora and vegetation of the Asir Mountain of South West, Saudi Arabia. Afr. J. Biotechnol. 2010, 9, 5550–5559.
- Almazroui, M. Changes in temperature trends and extremes over Saudi Arabia for the period 1978–2019. Adv. Meteorol. 2020, 2020, 8828421.
- Almazroui, M.; Nazrul Islam, M.; Athar, H.; Jones, P.D.; Rahman, M.A. Recent climate change in the Arabian Peninsula: Annual rainfall and temperature analysis of Saudi Arabia for 1978–2009. Int. J. Climatol. 2012, 32, 953–966.
- Almazroui, M.; Islam, M.N.; Jones, P.D.; Athar, H.; Rahman, M.A. Recent climate change in the Arabian Peninsula: Seasonal rainfall and temperature climatology of Saudi Arabia for 1979–2009. Atmos. Res. 2012, 111, 29–45.
- Al-Eisawi, D.M.; Al-Ruzayza, S. The flora of holy Mecca district, Saudi Arabia. Int. J. Biodivers. Conserv. 2015, 7, 173–189.
- Rahman, M.A.; Mossa, J.S.; Al-Said, M.S.; Al-Yahya, M.A. Medicinal plant diversity in the flora of Saudi Arabia 1: A report on seven plant families. Fitoterapia 2004, 75, 149–161.
- Shawky, R.A.; Alzamel, N.M. Survey on medicinal plants in the flora of Al Riyadh Region, Saudi Arabia. EurAsian J. BioSci. 2020, 14, 3795–3800.
- Al-Khazan, M.M.; Al-Zlabani, R.M. Toxic materials phytoremediation potential of four common trees in Saudi Arabia: A review. Egypt. J. Exp. Biol. (Bot.) 2019, 15, 87–97.
- Wildlife, N.c.f. Plant Diversity. Available online: https://www.ncw.gov.sa/Ar/Wildlife/Biodiversity/Pages/MarineEnvironments.aspx (accessed on 4 July 2022).
- Sher, H.; Aldosari, A. Overview on the ecological and geographical appraisal of important medicinal and aromatic plants: An endangered component in the flora of Saudi Arabia. Sci. Res. Essays 2012, 7, 1639–1646.
- Al-Khulaidi, A.W.A.; Al-Sagheer, N.A.; Al-Turki, T.; Filimban, F. Inventory of most rare and endangeredplant species in Albaha region, Saudi Arabia. IJBPAS 2018, 7, 443–460.
- Khalik, K.A.; El-Sheikh, M.; El-Aidarous, A. Floristic diversity and vegetation analysis of wadi Al-Noman, Mecca, Saudi Arabia. Turk. J. Bot. 2013, 37, 894–907.
- Abd El-Ghani, M.M. Vegetation along a transect in the Hijaz mountains (Saudi Arabia). J. Arid Environ. 1996, 32, 289–304.
- Batanouny, K.H.; Baeshin, N.A. Studies on the flora of Arabia II. The Medina-Badr Road. Saudi Arabia. Bull. Fac. Sci. KAU Jeddah 1982, 6, 1–26.
- Alfarhan, A.H.; Al-Turki, T.A.; Basahy, A.Y. Flora of Jizan region. Final Rep. Support. King Abdulaziz City Sci. Technol. 2005, 1, 545.
- Al-Sodany, Y.M.; Salih, A.B.; Mosallam, H.A. Medicinal plants in Saudi Arabia: I. Sarrwat Mountains at Taif, KSA. AJPS 2013, 6, 134–145.
- Abo-Hassan, A.A. Rangeland Management in Saudi Arabia. Rangel. Arch. 1981, 3, 51–53.
- Al-Sherif, E.A.; Ayesh, A.M.; Rawi, S.M. Floristic composition, life form and chorology of plant life at Khulais region, Western Saudi Arabia. Pak. J. Bot 2013, 45, 29–38.
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3670–3695.
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000, 17, 215–234.
- Al-Essa, M.A.; Al-Mehaidib, A.; Al-Gain, S. Parental awareness of liver disease among children in Saudi Arabia. Ann. Saudi Med. 1998, 18, 79–81.
- Zaid, H.; Rayan, A.; Said, O.; Saad, B. Cancer treatment by Greco-Arab and Islamic herbal medicine. Open Nutraceuticals J. 2010, 3, 203–212.
- El-Seedi, H.R.; Khalifa, S.A.M.; Yosri, N.; Khatib, A.; Chen, L.; Saeed, A.; Efferth, T.; Verpoorte, R. Plants mentioned in the Islamic Scriptures (Holy Qur’ân and Ahadith): Traditional uses and medicinal importance in contemporary times. J. Ethnopharmacol. 2019, 243, 112007.
- AbdElRahman, H.F.; Skaug, N.; Francis, G.W. In vitro antimicrobial effects of crude miswak extracts on oral pathogens. In Vitro 2002, 10, 15–21.
- An-Nasai, A. English Translation of Sunan An-Nasâ’i; Darussalam: Riyadh, Saudi Arabia, 2007; Volume 1.
- Leonti, M.; Verpoorte, R. Traditional Mediterranean and European herbal medicines. J. Ethnopharmacol. 2017, 199, 161–167.
- Aumeeruddy, M.Z.; Zengin, G.; Mahomoodally, M.F. A review of the traditional and modern uses of Salvadora persica L.(Miswak): Toothbrush tree of Prophet Muhammad. J. Ethnopharmacol. 2018, 213, 409–444.
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901.
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776.
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204.
- Toghueo, R.M.K. Anti-leishmanial and Anti-inflammatory Agents from Endophytes: A Review. Nat. Prod. Bioprospect. 2019, 9, 311–328.
- Gadallah, A.S.; Yousuf, S.; Jabeen, A.; Swilam, M.M.; Khalifa, S.A.M.; El-Seedi, H.R.; Choudhary, M.I. Anti-inflammatory principles from Tamarix aphylla L.: A bioassay-guided fractionation study. Molecules 2020, 25, 2994.
- Alsayari, A.; Wahab, S. Genus Ziziphus for the treatment of chronic inflammatory diseases. Saudi J. of Biol. Sci. 2021, 28, 6897–6914.
- Jachak, S.M.; Saklani, A. Challenges and opportunities in drug discovery from plants. Curr. Sci. 2007, 92, 1251–1257.
- Morsi, D.S.; El-Nabi, S.H.; Elmaghraby, M.A.; Abu Ali, O.A.; Fayad, E.; Khalifa, S.A.M.; El-Seedi, H.R.; El-Garawani, I.M. Anti-proliferative and immunomodulatory potencies of cinnamon oil on Ehrlich ascites carcinoma bearing mice. Sci. Rep. 2022, 12, 1–18.
- Zayed, M.; El-Garawani, I.M.; El-Sabbagh, S.M.; Amr, B.; Alsharif, S.M.; Tayel, A.A.; AlAjmi, M.F.; Ibrahim, H.; Shou, Q.; Khalifa, S.A.M. Structural Diversity, LC-MS-MS Analysis and Potential Biological Activities of Brevibacillus laterosporus Extract. Metabolites 2022, 12, 1102.
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol. Res. 2019, 139, 126–140.
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412.
- Elsharkawy, E.; Alshathly, M.; Helal, M. Anti-inflammatory and chemical composition of two plants Family Asteraceae growing in Saudi Arabia. J. Chem 2014, 8, 157–162.
- Elsharkawy, E.; Elshathely, M.; Jaleel, G.A.; Al-Johar, H.I. Anti-inflammatory effects of medicinal plants mixture used by Bedouin people in Saudi Arabia. Herba Pol. 2013, 59, 76–87.
- Mohamed, G.A.; Ibrahim, S.R.M.; Al-Musayeib, N.M.; Ross, S.A. New anti-inflammatory flavonoids from Cadaba glandulosa Forssk. Arch. Pharmacal Res. 2014, 37, 459–466.
- Shehata, I.A.; El-harshany, E.; Abdallah, H.M.; Esmat, A.; Abdel-Sattar, E.A. Anti-inflammatory activity of Kleinia odora. Eur. J. Integr. Med. 2018, 23, 64–69.
- Hamam, F.; Eldalo, A.; Abdallah, Q.; Al-Deeb, I.; Alzahrani, S.; Alwagdani, A.; Alotaibi, A.; Nasr, A.-R.; Gouda, Y.; Mohamed, K. Pharmacological activities of a novel plant species, Huernia Sp. Nov. aff. Boleana growing in the high mountains of southwest Saudi Arabia. Mol. Med. Rep. 2018, 17, 6059–6067.
- Albadawi, D.A.; Mothana, R.A.; Khaled, J.M.; Ashour, A.E.; Kumar, A.; Ahmad, S.F.; Al-Said, M.S.; Al-Rehaily, A.J.; Almusayeib, N.M. Antimicrobial, anticancer, and antioxidant compounds from Premna resinosa growing in Saudi Arabia. Pharm. Biol. 2017, 55, 1759–1766.
- Abbas, F.A.; Al-Massarany, S.M.; Khan, S.; Al-Howiriny, T.A.; Mossa, J.S.; Abourashed, E.A. Phytochemical and biological studies on Saudi Commiphora opobalsamum L. Nat. Prod. Res. 2007, 21, 383–391.
- Al-Jaber, N.A. Phytochemical and biological studies of Sisymbrium irio L. Growing in Saudi Arabia. J. Saudi Chem. Soc. 2011, 15, 345–350.
- Al-Musayeib, N.; Perveen, S.; Fatima, I.; Nasir, M.; Hussain, A. Antioxidant, anti-glycation and anti-inflammatory activities of phenolic constituents from Cordia sinensis. Molecules 2011, 16, 10214–10226.
- Hawas, U.W.; Abou El-Kassem, L.T.; Shaher, F.; Al-Farawati, R. In vitro inhibition of Hepatitis C virus protease and antioxidant by flavonoid glycosides from the Saudi costal plant Sarcocornia fruticosa. Nat. Prod. Res. 2019, 33, 3364–3371.
- Al-Haidari, R.A.; Al-Oqail, M.M. New benzoic acid derivatives from Cassia italica growing in Saudi Arabia and their antioxidant activity. Saudi Pharm. J. 2020, 28, 1112–1117.
- Dal Piaz, F.; Bader, A.; Malafronte, N.; D’Ambola, M.; Petrone, A.M.; Porta, A.; Hadda, T.B.; De Tommasi, N.; Bisio, A.; Severino, L. Phytochemistry of compounds isolated from the leaf-surface extract of Psiadia punctulata (DC.) Vatke growing in Saudi Arabia. Phytochemistry 2018, 155, 191–202.
More
