Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Liu Zhihui.
Lung cancer is one of the 10 most common cancers in the world, which seriously affects the normal life and health of patients. The mechanisms of action of the occurrence and development of lung cancer have not been fully clarified. As a new type of gas signal molecule, hydrogen sulfide (H2S) has received great attention for its physiological and pathological roles in mammalian cells. It has been found that H2S is widely involved in the regulation of the respiratory system and digestive system, and plays an important role in the occurrence and development of lung cancer.
- hydrogen sulfide
- lung cancer
- signaling pathway
- molecular mechanism
1. Introduction
Lung cancer is a common malignant tumor that seriously threatens human life and health [1]. Its morbidity and mortality are at the top of the list in most countries [2]. As the early clinical symptoms of lung cancer are not obvious, most of the patients are diagnosed with a serious condition. According to the pathological classification, lung cancer can be divided into small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC). SCLC is the most malignant type of lung cancer, with an incidence of about 15–20%. NSCLC is mainly divided into squamous cell carcinoma, adenocarcinoma and large cell carcinoma, with an incidence about 80–85% [3,4,5][3][4][5]. At present, the main strategies for the treatment of lung cancer are surgery combined with radiotherapy and chemotherapy; however, the side effects are obvious. Therefore, it is urgent to develop novel methods for the treatment of lung cancer.
H2S is a new type of gas signal molecule after nitric oxide (NO) and carbon monoxide (CO) [6], and it is a colorless, flammable, and water-soluble gas with a rotten egg smell [7,8,9][7][8][9]. H2S is mainly produced by cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) [10,11,12][10][11][12]. The third enzyme, 3-mercaptopyruvate sulfurtransferase (3-MST), can also generate endogenous H2S in the presence of a reductant using 3-mercaptopyruvate (3-MP) as substrate [13,14][13][14]. In addition, tobacco cigarette smoke, industrial gases, NaHS, GYY4137, AP39, etc., may produce exogenous H2S. H2S is reported to be involved in the development of many diseases [15] and it has both pro-apoptotic and anti-apoptotic effects in cultured cells [16,17][16][17]. H2S can participate in the occurrence and development of tumors through the mitogen-activated protein kinase-extracellular signal-regulated kinase (MAPK-ERK1/2) pathway, endoplasmic reticulum stress, and ion channels [18,19][18][19]. H2S has been shown to be involved in the occurrence and development of lung cancer [20].
2. Synthesis and Metabolism of H2S in Lung Cancer
2.1. CSE
H2S is mainly produced by CBS and CSE [10,11,12][10][11][12]. The third enzyme, 3-MST, can also promote the production of endogenous H2S from 3-MP in the presence of reducing agents [13,14][13][14]. 3-MST exists in both mitochondria and cytoplasm, while CBS and CSE mainly exist in the cytoplasm [21]. Analysis of NSCLC biopsies and adjacent non-tumor tissues showed selectively high levels of endogenous H2S-producing enzymes, namely CBS, CSE, and 3-MST [22,23][22][23].
CSE is a homo-tetramer composed of pyridoxal 5′-phosphate (PLP)-bound 45 kDa subunits, and is the second enzyme to form H2S in the transsulfuration pathway. CSE mainly decomposes cysteine, a byproduct of CBS, into cysteine, α-ketobutyrate, and ammonia. Like CBS, CSE can also decompose cysteine and produce H2S (Figure 1). The PLP-CSE interaction is required for enzymic activity [23]. Hypoxia is a typical feature of solid tumors, including NSCLC [24]. CSE activity is a key driver of the transsulfuration pathway, cysteine catabolism, and H2S production. Tumor angiogenesis is induced by H2S mediated via hypoxia. H2S can increase the ability of endothelial cell invasion and duct formation [22]. H2S also plays a protective role in tumor-induced oxidative stress [25].
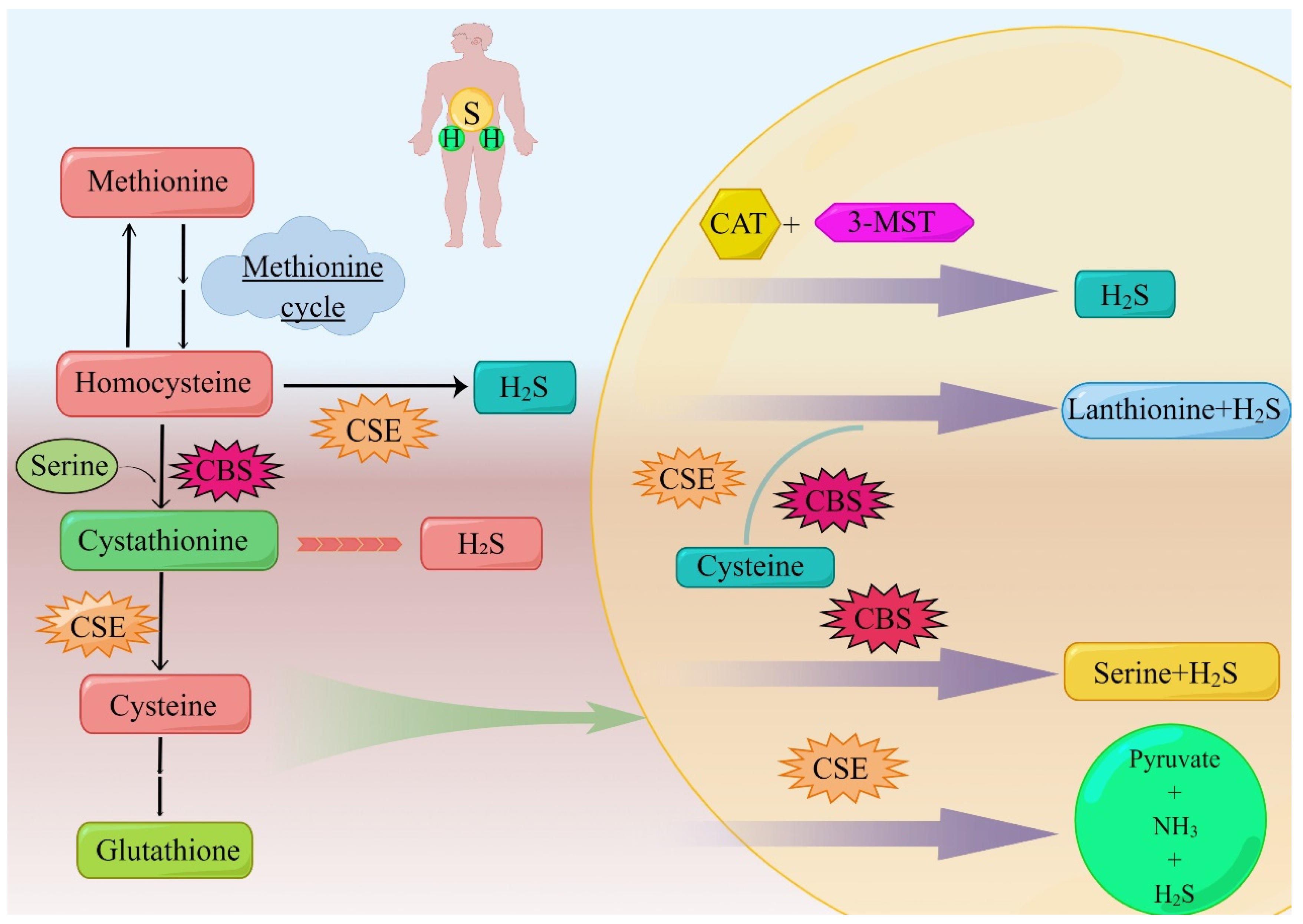
Figure 1. The body produces H2S through the anti-sulfide pathway. The sulfur transfer pathway plays an important role in redox regulation and cellular sulfur metabolism. The sulfur in this pathway is transferred from homocysteine to cysteine via mesocysteine, which is the only pathway for the endogenous production of cysteine in mammals. Methionine is converted to homocysteine in a reversible two-step process catalyzed by SAM and S-adenosine homocystease (not shown). Homocysteine is introduced into serine to produce cystathionine under the mediation of CBS, and this step can produce H2S. CSE can use homocysteine or cystathionine as substrate, the former to produce H2S, the latter to produce cysteine to continue the synthesis of H2S mediated by CSE, CBS, and 3-MST. Additionally, this part of the cysteine is involved in the synthesis of glutathione. 3-MST can cooperate with CAT to participate in the generation of H2S. CSE and CBS can mediate the generation of H2S with the addition of serine together, and they can also separately mediate the generation of H2S. Abbreviations; CBS: cystathionine β-synthase; CSE: cystathionine γ-lyase; 3-MST: 3-mercaptopyruvate sulfurtransferase; H2S: hydrogen sulfide; SAM: S-adenosylmethionine; GSH: glutathione; CAT: cysteine transaminase. (By Figdraw).
The typical role of CSE in the transsulfuration pathway is to cleave cysteine to form cysteine, ammonia, and α-ketobutyrate. Different from CBS, only homocysteine is used as the substrate of CSE in the formation of H2S [14]. Due to the low specificity of the CSE substrate, cystathionine, cysteine, and homocysteine can be regulated in the same binding capsule, where they compete with PLP to form Schiff base, while CBS has no binding site for PLP; therefore, H2S produced by CSE is more sensitive to homocysteine [12].
The protein and mRNA levels of CSE in tumor tissues are higher than in adjacent tissues. Compared with the corresponding levels in normal lung epithelial cell line BEAS-2B, NSCLC cell lines (A549 and 95D) showed the selective up-regulation of protein and mRNA expression of all three H2S-producing enzymes. It can be concluded that NSCLC cells selectively over-express CSE, thus inducing H2S production and promoting cell proliferation, migration, and invasion [22]. However, some studies have shown that compared with CBS, the clinical correlation between the expression of CSE in tumors and the prognosis of patients is not significant. There is no difference in clinical outcomes between high and low CSE expression in most cancers [26].
2.2. CBS
CBS is a homologous tetrameric enzyme with about 63 kDa subunits, which binds to two cofactors, PLP and heme [27]. The first and committed step in the transsulfuration pathway of the catalytic conversion of H2S by CBS is to use homocysteine instead of serine to form cystathionine and water. When the substrate is cysteine instead of serine, the products of the reaction are cysteine and H2S. CBS also catalyzes other reactions of cysteine to H2S [14]. Through the gene knockout experiment, it can be concluded that CSE is necessary for the synthesis of cysteine through sulfur transfer, and its gene destruction deprives CBS of one of the substrates needed to produce H2S. Thus, the disruption of CSE genes affects CSE- and CBS-dependent H2S synthesis and reduces H2S production. In contrast, disruption of the CBS gene results in the accumulation of homocysteine, a substrate for H2S production by CSE [10,14][10][14].
CBS exists mainly in the cytoplasm under normal physiological conditions [21]; however, CBS can be transferred to mitochondria in response to hypoxia or ischemia, a process that is partly the result of Lon protease regulating mitochondrial CBS stability [28]. Several studies have shown that hypoxia can be a condition that induces CBS translocation into the mitochondria. In fact, CBS can be detected in both the cytosolic and mitochondrial parts of HCT116 cells and A2780 cells [29,30][29][30]. The translocation of CBS to mitochondria may have important implications for the regulation of cancer cell bioenergy and survival [31]. It may be the result of threonine acylation that CBS enters the nucleus [32].
NSCLC cells selectively over-express CBS, which induces H2S production and promotes cell proliferation, migration, and invasion [22]. For example, in human non-small-cell lung adenocarcinoma, Western blot analysis of tumor tissue compared with normal adjacent lung tissue (n = 20) showed that CBS protein is significantly increased about five-fold in tumor homogenate; furthermore, these tissues also produce approximately two times as much H2S as their surrounding normal tissues [33]. Increased levels of CBS protein or mRNA have also been reported in two different collections of lung cancer clinical specimens [8]. Hypoxia, glucose deprivation, and hydrogen peroxide treatment have been applied to various HCC and breast cancer cells to generate repaired cells, which are partially resistant to subsequent injury and exhibit the up-regulation of CBS; therefore, in this experimental model, oxidative stress seems to be the most important factor leading to the up-regulation of CBS [34,35][34][35]. CBS mRNA and protein levels are slightly up-regulated in chemically hypoxic A549 lung cancer cells treated with cobalt chloride [22]. Lung adenocarcinoma cell lines A549, H522, H1944, Calu-6 cells, A549, and 95D cells are used to examine the functional role of CBS. The results show that the expression of CBS in cancer cell lines is significantly higher than that in the untransformed lung epithelial cell line Beas2B control [8,22,33][8][22][33]. Studies have shown that CBS mRNA is induced in response to the transcription factor Nrf2 [36], and CBS protein levels are regulated by both transcriptional and post-transcriptional processes, including ubiquitination [31].
2.3. 3-MST
3-MST is a 33 kDa zinc-dependent enzyme and H2S-producing enzyme. As early as 1959, Hylin and Wood confirmed that 3-MST could produce polysulfides [37]. Kamoun thought that 3-MST had the ability to produce H2S as early as 2004. 3-MST is a PLP-independent enzyme that catalyzes L-cysteine to form H2S. In addition, 3-MST can bind to cysteine aminotransferase in the presence of α-ketoglutarate [38].
3-MST is structurally expressed in all kinds of somatic cells, as well as various cancer cells. Wrobel’s team has performed related earlier studies on the expression of 3-MST in cancer cells: in human tumor cell lines (U373 astrocytoma cell line; SH-SY5Y neuroblastoma cell line; and two melanoma cells lines, A375 and WM35), they found a large amount of 3-MST expression and enzymatic activity. The expression and activity of 3-MST in these cell lines are significantly higher than those of CSE, so it can be concluded that 3-MST is a more important source of H2S [39,40][39][40]. Western blotting indicates [33] that 3-MST expression is either considerably greater or marginally higher in human lung cancer tumors compared with adjacent non-cancer tissues. However, the expression of 3-MST in human papillary thyroid carcinoma tumors is not different from that in surrounding non-cancer tissues [41], and the expression level of 3-MST in renal cell carcinoma tumors is highly variable [42]. At present, a variety of lung adenocarcinoma cell lines (A549 H522~H1944) have been proven to have 3-MST expression/catalytic activity [33,43][33][43].
One of the characteristics of lung cancer is angiogenesis, and this process can supply the oxygen and nutrients required for the growth of lung cancer cells; furthermore, it also creates the potential for tumor spread. It is reported that H2S has the ability to promote new blood vessels [44,45][44][45]. Related studies have shown that 3-MST-derived H2S produced by endothelial cells plays a certain role in vasodilation, endothelial cell proliferation, migration, and angiogenesis, especially under hypoxic conditions [13,46,47,48][13][46][47][48]. Some studies have shown that silent 3-MST can reduce the proliferation rate of A549 cells (human lung adenocarcinoma cell line) and decrease the repair rate of mitochondrial DNA [37]. To summarize, 3-MST participates in the generation and development of lung cancer and plays an important role. Furthermore, researchers compare CSE, CBS, and 3-MST in Table 1.
Table 1. Abbreviations: CSE: cystathionine γ-lyase; EMT: epithelial–mesenchymal transition; H2S: hydrogen sulfide; CBS: cystathionine β-synthase; NSCLC: non-small-cell lung cancer; 3-MST: 3-mercaptopyruvate sulfurtransferase.
| Table | Location | Transfer | Expression and Activity | The Effect after Inhibition |
|---|---|---|---|---|
| CSE | Cytoplasm [21] | CSE can be transferred from the cytoplasm to the mitochondria in response to cellular stress, such as increased intracellular Ca2+ levels [29,30,49][29][30][49]. | The clinical correlation between CSE expression in tumor and patient prognosis was not significant. In most cancers, there was no difference in clinical outcomes between high and low CSE expression [26]. | Silencing H2S synthase, especially CSE, inhibits the EMT process in NSCLC cells [22]. |
| CBS | Cytoplasm [21] | Hypoxia may be a condition that leads to the translocation of CBS into mitochondria, which is of great significance for the regulation of biological energy and survival in cancer cells [28,31][28][31]. | Oxidative stress seems to be the most important factor leading to the upregulation of CBS. Cells with overexpression of CBS show higher metabolism, proliferation, aggressiveness, dedifferentiated dry state, chemotherapy resistance, and immune cell resistance [8,22,33][8][22][33]. | CBs-derived H2S has been identified as a target for tumor growth factors and anticancer drugs, and loss of CBS blocks the density and curl of CD31-positive blood vessels between tumor tissues, indicating reduced tumor angiogenesis [29]. |
| 3-MST | Mitochondria and cytoplasm [21] | Lack of coverage. | Cancer cell lines with 3-MST expression/catalytic activity include various lung adenocarcinoma cell lines. 3-MST is involved in the occurrence and development of lung cancer and plays an important role. 3-MST can play an effective role in the treatment of lung cancer; however, its mechanism is not clear and more research is needed [48]. | H2S synthesis decreased after 3-MST inhibition [50,51,52][50][51][52]. |
2.4. Catabolism of H
2
S in Lung Cancer
CSE and CBS are primarily expressed in human airway smooth muscle cells (SMCs), pulmonary blood vessels and endothelial cells, and the airway SMCs of mouse lung. Some studies have shown a higher expression of CSE in the airway and peripheral lung tissue of rat pulmonary blood vessels [53]. The primary steps in H2S catabolism include exhalation, lung ventilation, methylation modification, and oxidation. Large quantities of H2S cause the mitochondria to generate thiosulfate, which is converted to sulfate by rhodanese. Thiol S-methyltransferase, on the other hand, catalyzes the methylation of H2S in the cytoplasm, where it is transformed into methanethiol and dimethyl sulfide [23]. Methanethiol is converted into dimethyl sulfide by S-methyltransferase. In conclusion, H2S may be quickly expelled from the body as a gas in the respiratory system and can be eliminated in the urine as sulfate and thiosulfate.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Rafei, H.; El-Bahesh, E.; Finianos, A.; Nassereddine, S.; Tabbara, I. Immune-based Therapies for Non-small Cell Lung Cancer. Anticancer Res. 2017, 37, 377–387.
- Rodriguez-Canales, J.; Parra-Cuentas, E.; Wistuba, I.I. Diagnosis and Molecular Classification of Lung Cancer. Cancer Treat. Res. 2016, 170, 25–46.
- Zheng, M. Classification and Pathology of Lung Cancer. Surg. Oncol. Clin. N. Am. 2016, 25, 447–468.
- Collins, L.G.; Haines, C.; Perkel, R.; Enck, R.E. Lung cancer: Diagnosis and management. Am. Fam. Physician 2007, 75, 56–63.
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071.
- Szabo, C.; Papapetropoulos, A. International Union of Basic and Clinical Pharmacology. CII: Pharmacological Modulation of H(2)S Levels: H(2)S Donors and H(2)S Biosynthesis Inhibitors. Pharmacol. Rev. 2017, 69, 497–564.
- Russo, A.; Saide, A.; Cagliani, R.; Cantile, M.; Botti, G.; Russo, G. rpL3 promotes the apoptosis of p53 mutated lung cancer cells by down-regulating CBS and NFκB upon 5-FU treatment. Sci. Rep. 2016, 6, 38369.
- Wang, R. Hydrogen sulfide: The third gasotransmitter in biology and medicine. Antioxid. Redox Signal. 2010, 12, 1061–1064.
- Chiku, T.; Padovani, D.; Zhu, W.; Singh, S.; Vitvitsky, V.; Banerjee, R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 2009, 284, 11601–11612.
- Porter, T.D.; Beck, T.W.; Kasper, C.B. NADPH-cytochrome P-450 oxidoreductase gene organization correlates with structural domains of the protein. Biochemistry 1990, 29, 9814–9818.
- Singh, S.; Padovani, D.; Leslie, R.A.; Chiku, T.; Banerjee, R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 2009, 284, 22457–22466.
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009, 146, 623–626.
- Kabil, O.; Banerjee, R. Enzymology of H2S biogenesis, decay and signaling. Antioxid. Redox Signal. 2014, 20, 770–782.
- Toliver-Kinsky, T.; Cui, W.; Törö, G.; Lee, S.J.; Shatalin, K.; Nudler, E.; Szabo, C. H(2)S, a Bacterial Defense Mechanism against the Host Immune Response. Infect. Immun. 2019, 87, e00272-18.
- Hu, L.F.; Wong, P.T.; Moore, P.K.; Bian, J.S. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J. Neurochem. 2007, 100, 1121–1128.
- Taniguchi, S.; Kang, L.; Kimura, T.; Niki, I. Hydrogen sulphide protects mouse pancreatic β-cells from cell death induced by oxidative stress, but not by endoplasmic reticulum stress. Br. J. Pharmacol. 2011, 162, 1171–1178.
- Zhong, G.Z.; Li, Y.B.; Liu, X.L.; Guo, L.S.; Chen, M.L.; Yang, X.C. Hydrogen sulfide opens the KATP channel on rat atrial and ventricular myocytes. Cardiology 2010, 115, 120–126.
- Gobbi, G.; Ricci, F.; Malinverno, C.; Carubbi, C.; Pambianco, M.; de Panfilis, G.; Vitale, M.; Mirandola, P. Hydrogen sulfide impairs keratinocyte cell growth and adhesion inhibiting mitogen-activated protein kinase signaling. Lab. Investig. 2009, 89, 994–1006.
- Wu, D.; Wang, H.; Teng, T.; Duan, S.; Ji, A.; Li, Y. Hydrogen sulfide and autophagy: A double edged sword. Pharmacol. Res. 2018, 131, 120–127.
- Donnarumma, E.; Trivedi, R.K.; Lefer, D.J. Protective Actions of H2S in Acute Myocardial Infarction and Heart Failure. Compr. Physiol. 2017, 7, 583–602.
- Wang, M.; Yan, J.; Cao, X.; Hua, P.; Li, Z. Hydrogen sulfide modulates epithelial-mesenchymal transition and angiogenesis in non-small cell lung cancer via HIF-1α activation. Biochem. Pharmacol. 2020, 172, 113775.
- Murphy, B.; Bhattacharya, R.; Mukherjee, P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019, 33, 13098–13125.
- Yip, C.; Blower, P.J.; Goh, V.; Landau, D.B.; Cook, G.J.R. Molecular imaging of hypoxia in non-small-cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 956–976.
- Yan, S.K.; Chang, T.; Wang, H.; Wu, L.; Wang, R.; Meng, Q.H. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2006, 351, 485–491.
- Silver, D.J.; Roversi, G.A.; Bithi, N.; Wang, S.Z.; Troike, K.M.; Neumann, C.K.; Ahuja, G.K.; Reizes, O.; Brown, J.M.; Hine, C.; et al. Severe consequences of a high-lipid diet include hydrogen sulfide dysfunction and enhanced aggression in glioblastoma. J. Clin. Investig. 2021, 131, e138276.
- Meier, M.; Janosik, M.; Kery, V.; Kraus, J.P.; Burkhard, P. Structure of human cystathionine beta-synthase: A unique pyridoxal 5’-phosphate-dependent heme protein. EMBO J. 2001, 20, 3910–3916.
- Teng, H.; Wu, B.; Zhao, K.; Yang, G.; Wu, L.; Wang, R. Oxygen-sensitive mitochondrial accumulation of cystathionine β-synthase mediated by Lon protease. Proc. Natl. Acad. Sci. USA 2013, 110, 12679–12684.
- Szabo, C.; Coletta, C.; Chao, C.; Módis, K.; Szczesny, B.; Papapetropoulos, A.; Hellmich, M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 12474–12479.
- Bhattacharyya, S.; Saha, S.; Giri, K.; Lanza, I.R.; Nair, K.S.; Jennings, N.B.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Basal, E.; Weaver, A.L.; et al. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS ONE 2013, 8, e79167.
- Ascenção, K.; Szabo, C. Emerging roles of cystathionine β-synthase in various forms of cancer. Redox Biol. 2022, 53, 102331.
- Kabil, O.; Zhou, Y.; Banerjee, R. Human cystathionine beta-synthase is a target for sumoylation. Biochemistry 2006, 45, 13528–13536.
- Szczesny, B.; Marcatti, M.; Zatarain, J.R.; Druzhyna, N.; Wiktorowicz, J.E.; Nagy, P.; Hellmich, M.R.; Szabo, C. Inhibition of hydrogen sulfide biosynthesis sensitizes lung adenocarcinoma to chemotherapeutic drugs by inhibiting mitochondrial DNA repair and suppressing cellular bioenergetics. Sci. Rep. 2016, 6, 36125.
- Sanokawa-Akakura, R.; Ostrakhovitch, E.A.; Akakura, S.; Goodwin, S.; Tabibzadeh, S. A H2S-Nampt dependent energetic circuit is critical to survival and cytoprotection from damage in cancer cells. PLoS ONE 2014, 9, e108537.
- Ostrakhovitch, E.A.; Akakura, S.; Sanokawa-Akakura, R.; Goodwin, S.; Tabibzadeh, S. Dedifferentiation of cancer cells following recovery from a potentially lethal damage is mediated by H2S-Nampt. Exp. Cell Res. 2015, 330, 135–150.
- Hourihan, J.M.; Kenna, J.G.; Hayes, J.D. The gasotransmitter hydrogen sulfide induces nrf2-target genes by inactivating the keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between cys-226 and cys-613. Antioxid. Redox Signal. 2013, 19, 465–481.
- Augsburger, F.; Szabo, C. Potential role of the 3-mercaptopyruvate sulfurtransferase (3-MST)-hydrogen sulfide (H(2)S) pathway in cancer cells. Pharmacol. Res. 2020, 154, 104083.
- Kimura, H. Hydrogen sulfide: Its production, release and functions. Amino Acids 2011, 41, 113–121.
- Jurkowska, H.; Placha, W.; Nagahara, N.; Wróbel, M. The expression and activity of cystathionine-γ-lyase and 3-mercaptopyruvate sulfurtransferase in human neoplastic cell lines. Amino Acids 2011, 41, 151–158.
- Bronowicka-Adamska, P.; Bentke, A.; Wróbel, M. Hydrogen sulfide generation from l-cysteine in the human glioblastoma-astrocytoma U-87 MG and neuroblastoma SHSY5Y cell lines. Acta Biochim. Pol. 2017, 64, 171–176.
- Xu, Y.; Ma, N.; Wei, P.; Zeng, Z.; Meng, J. Expression of hydrogen sulfide synthases and Hh signaling pathway components correlate with the clinicopathological characteristics of papillary thyroid cancer patients. Int. J. Clin. Exp. Pathol. 2018, 11, 1818–1824.
- Breza, J., Jr.; Soltysova, A.; Hudecova, S.; Penesova, A.; Szadvari, I.; Babula, P.; Chovancova, B.; Lencesova, L.; Pos, O.; Breza, J.; et al. Endogenous H(2)S producing enzymes are involved in apoptosis induction in clear cell renal cell carcinoma. BMC Cancer 2018, 18, 591.
- Bai, Y.W.; Ye, M.J.; Yang, D.L.; Yu, M.P.; Zhou, C.F.; Shen, T. Hydrogen sulfide attenuates paraquat-induced epithelial-mesenchymal transition of human alveolar epithelial cells through regulating transforming growth factor-β1/Smad2/3 signaling pathway. J. Appl. Toxicol. 2019, 39, 432–440.
- Cai, W.J.; Wang, M.J.; Moore, P.K.; Jin, H.M.; Yao, T.; Zhu, Y.C. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc. Res. 2007, 76, 29–40.
- Köhn, C.; Dubrovska, G.; Huang, Y.; Gollasch, M. Hydrogen sulfide: Potent regulator of vascular tone and stimulator of angiogenesis. Int. J. Biomed. Sci. 2012, 8, 81–86.
- Coletta, C.; Módis, K.; Szczesny, B.; Brunyánszki, A.; Oláh, G.; Rios, E.C.; Yanagi, K.; Ahmad, A.; Papapetropoulos, A.; Szabo, C. Regulation of Vascular Tone, Angiogenesis and Cellular Bioenergetics by the 3-Mercaptopyruvate Sulfurtransferase/H2S Pathway: Functional Impairment by Hyperglycemia and Restoration by DL-α-Lipoic Acid. Mol. Med. 2015, 21, 1–14.
- Tao, B.; Wang, R.; Sun, C.; Zhu, Y. 3-Mercaptopyruvate Sulfurtransferase, Not Cystathionine β-Synthase Nor Cystathionine γ-Lyase, Mediates Hypoxia-Induced Migration of Vascular Endothelial Cells. Front. Pharmacol. 2017, 8, 657.
- Abdollahi Govar, A.; Törő, G.; Szaniszlo, P.; Pavlidou, A.; Bibli, S.I.; Thanki, K.; Resto, V.A.; Chao, C.; Hellmich, M.R.; Szabo, C.; et al. 3-Mercaptopyruvate sulfurtransferase supports endothelial cell angiogenesis and bioenergetics. Br. J. Pharmacol. 2020, 177, 866–883.
- Fu, M.; Zhang, W.; Wu, L.; Yang, G.; Li, H.; Wang, R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. USA 2012, 109, 2943–2948.
- Nagahara, N.; Katayama, A. Post-translational regulation of mercaptopyruvate sulfurtransferase via a low redox potential cysteine-sulfenate in the maintenance of redox homeostasis. J. Biol. Chem. 2005, 280, 34569–34576.
- Wróbel, M.; Jurkowska, H. Menadione effect on l-cysteine desulfuration in U373 cells. Acta Biochim. Pol. 2007, 54, 407–411.
- Módis, K.; Asimakopoulou, A.; Coletta, C.; Papapetropoulos, A.; Szabo, C. Oxidative stress suppresses the cellular bioenergetic effect of the 3-mercaptopyruvate sulfurtransferase/hydrogen sulfide pathway. Biochem. Biophys. Res. Commun. 2013, 433, 401–407.
- Wang, P.; Zhang, G.; Wondimu, T.; Ross, B.; Wang, R. Hydrogen sulfide and asthma. Exp. Physiol. 2011, 96, 847–852.
More
