Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Ajitanshu Vedrtnam.
The requirement to counter carbon emissions is becoming urgent. Zeolitic Imidazolate Frameworks (ZIFs) have been extensively investigated for storing and separating gases, especially carbon dioxide. ZIFs are the subclass of metal-organic frameworks with analogous topology to inorganic porous zeolites. These are attractive candidates for several applications, including storage and separation of gases, due to their outstanding properties such as high CO2 uptake, large surface area, permanent porosity, high thermal stability, high chemical stability, etc.
- CO2 absorption
- characterisation
- metal-organic framework
- zeolites
- ZIF
1. Introduction
Zeolitic Imidazolate Framework (ZIF) is a nanoporous material consisting of transition metal ions and imidazolate-type linkers. ZIF is a porous crystal with a 3-D structure of tetrahedral bivalent metal ions linked with organic imidazolates. The bivalent metal ions comprise of transition metals such as Zn or Co atoms. ZIF structure is a combination of nodes (metal ions) which act as connecting points and linkers (organic ligands), which serve as bridging molecules. One of the most prominent and unique properties of ZIF is its hydrophobic characteristic. Researchers are working on the development of ZIF-Based methods for CO2 capture. Tan et al. have studied the effects of porosity and pore architecture, framework density and network topology, and chemical structure on the mechanical property (elastic modulus and hardness) of ZIFs [9][1]. Likewise, Phan et al. have reported the synthesis, structure, and CO2 capture properties of ZIFs. Their paper discusses a comprehensive list of ZIFs, their topology and pore metrics in detail [10][2]. Yan et al. performed an experimental study on CO2 capture by ZIFs slurry under normal pressure. The results showed that fine aperture, baffles, low superficial gas velocity, low sorption temperature, high regeneration temperature, and low regeneration pressure were favourable conditions for CO2 capture [11][3]. Song et al. have synthesized new composite material by ionic liquid functionalized ZIF and evaluated the CO2 adsorption behaviour at room temperature under low pressure. The results confirmed that altering porous material with ionic liquids could be an effective method to produce solid sorbent materials for CO2 adsorption [12][4]. Morris et al. conducted an experimental and computational investigation on five different series of ZIFs for CO2 capture. The results confirmed that CO2 uptake capacity is directly influenced by functionality’s symmetry and polarization ability [13][5]. Banerjee et al. developed a high throughput protocol for synthesizing ZIFs for CO2 capture. The results confirmed that these ZIFs structures have a tetrahedral framework, high thermal and chemical stability, high porosity and high selectivity for CO2 capture [14][6]. Bhattacharjee et al. have reviewed the synthesis, functionalization, and catalytic/adsorption application of ZIFs [15][7].
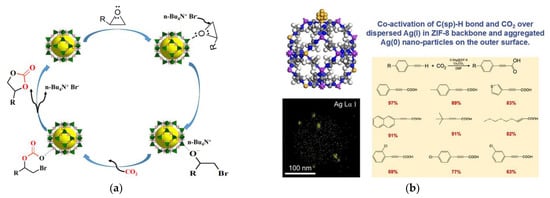
Similarly, Kukkar et al. have reviewed recent advances in synthetic techniques for ZIFs [16][8]. Lee et al. have reported a new approach to engineer nearly spherical ZIF crystal shape by using leaf-like pseudo-polymorph [17][9]. Qian et al. have provided a new approach to enhanced water stability in Zn doped ZIF for CO2 capture. The results revealed that Zn doped ZIF had shown better stability regarding crystal structure, morphology, surface area and CO2 uptake capacity [18][10]. Wang et al. have reported the synthesis of functional ZIF-templated porous carbon material for CO2 capture. The results confirmed that the functional group plays a vital role in improving the surface area, pore area and CO2 capture of corresponding porous carbon material [19][11]. Wu et al. demonstrated the synthesis of a porous carbon framework with high CO2 capture capacity by polyimide/ZIF composite aerogels. The results revealed an optimum CO2 capture capacity for carbonized aerogel at a loading of a certain percentage of ZIF [20][12]. Abdelhamid has reviewed the ZIFs for CO2 removal by adsorption, and catalysis processes such as cycloaddition, carboxylation, photocatalysis, electrocatalysis, etc. (Figure 1). The review has also promoted the industrial applications and commercialization of ZIFs [21][13].

2. ZIF Structure
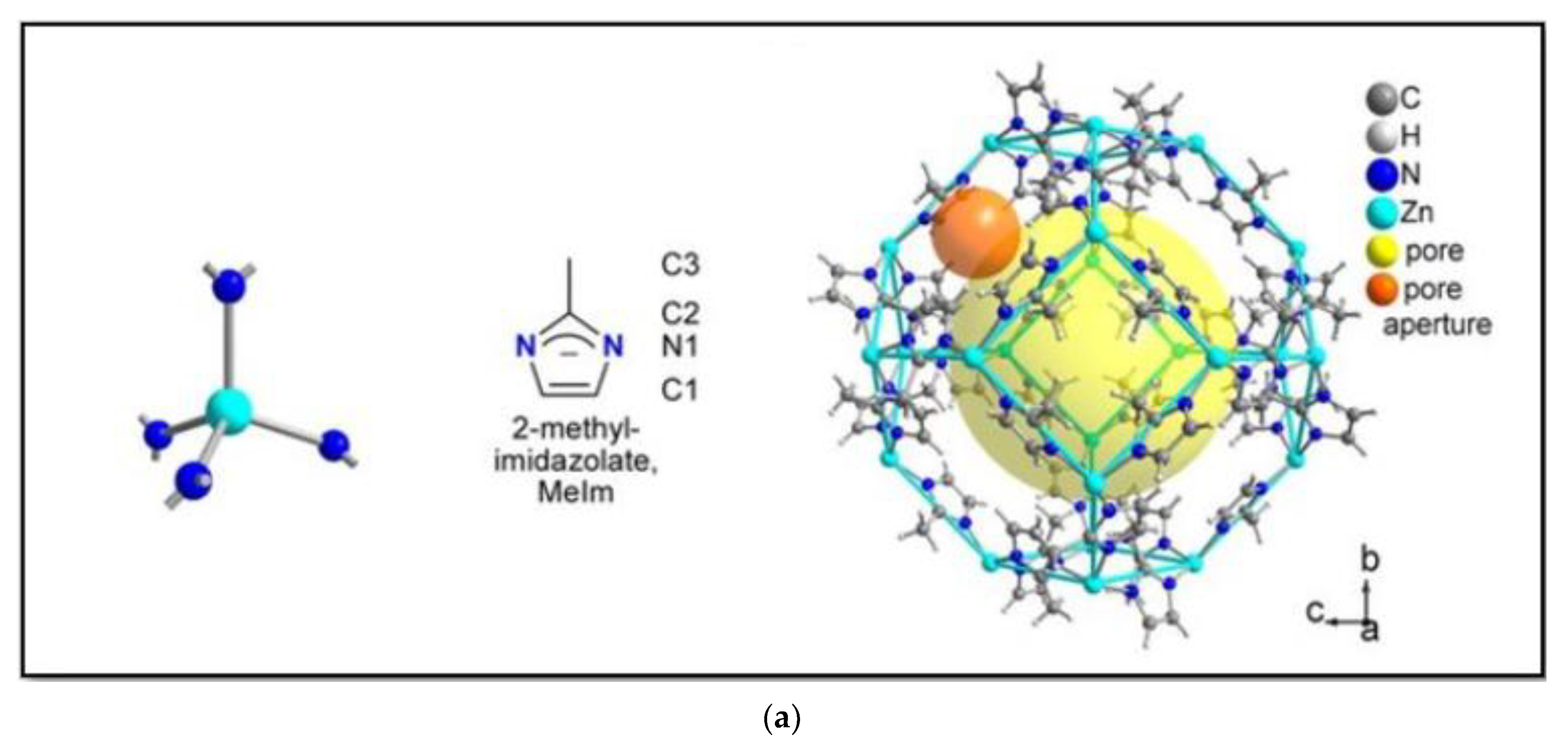
ZIFs have tetrahedral topologies and are created by connecting 4-coordinated transition metals (M) by imidazolates (Im) with an angle of 145°. The metal atoms are linked through N atoms by ditopic and functionalized Im links to construct neutral frameworks [22][14]. The M-Im-M (M = Zn or CO and Im = imidazolate) angle is responsible for synthesizing many ZIFs with zeolite-type -tetrahedral topologies. Several factors come into play when approaching the synthesis process of ZIFs, those being geometry, maintenance, functionality, conformation, compatibility, solubility, pH, temperature, etc. The structure of ZIFs mainly depends on the category of imidazolate and solvents used. In addition, the structural diversity in ZIFs is due to the use of functionalized imidazolate ligands in the synthesis process. ZIFs display zeolitic topologies (more than 105) such as sod, rho, or lta [21][13]. Figure 2 shows the unit cell and crystal structure of ZIF (Figure 2a) [23][15], along with the common structure of linker used to synthesis ZIFs (Figure 2b) [21][13].

3. Synthesis Techniques for ZIFs
There are a variety of synthetic techniques for ZIFs. Some of them are solvothermal (first used in 1995), electrochemistry (2003), hydrothermal (2004), microwave-assisted (2005), mechanochemical (2005), sonochemical (2006), template (2006), atomic layer deposition (2007), ionothermal (2009), spray dryer (2011), sol-gel (2011), supercritical (2012), flow chemistry (2015), etc. Furthermore, all these synthesis techniques can be divided into solvent technology, solid sorbent technology, membrane technology, and cryogenic distillation. The self-explanatory schematics of all these technologies are shown in Figure 3a–d.
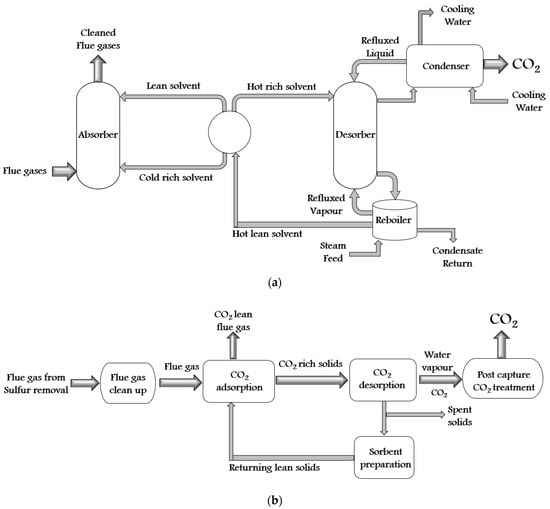
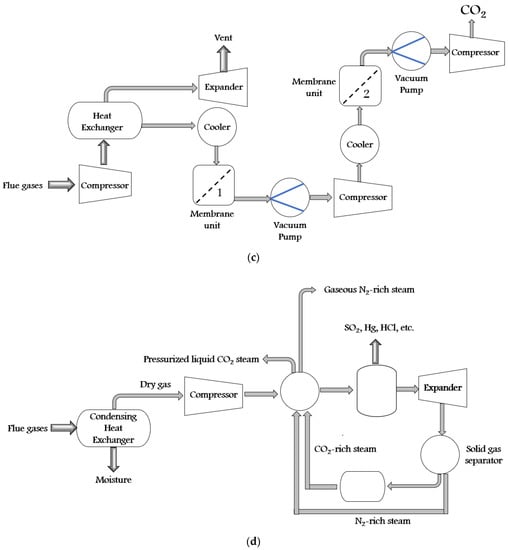
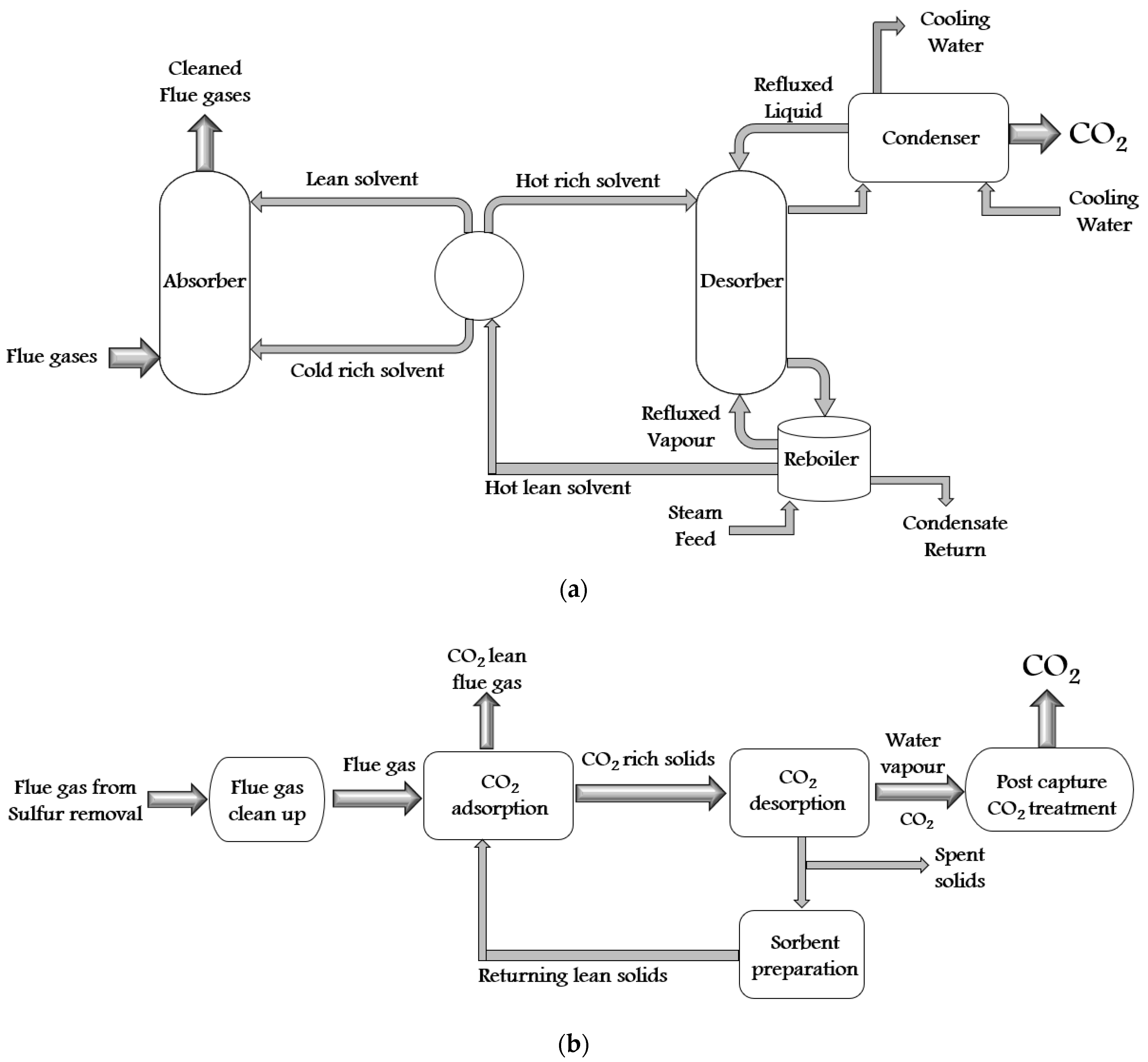
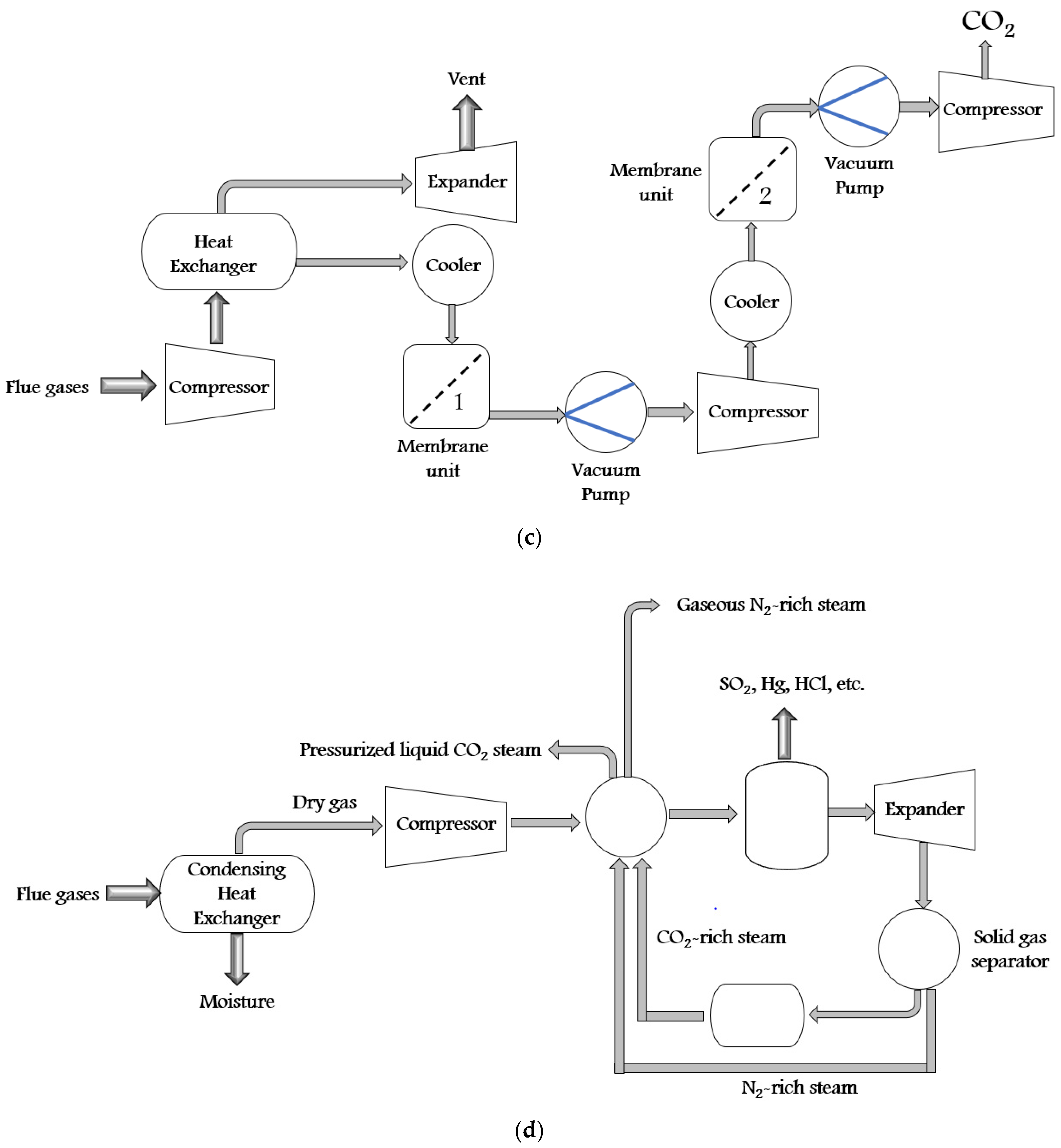
Figure 3. The schematic diagram of CO2 capture by (a) solvent technology, (b) solid sorbent technology, (c) membrane technology, and (d) cryogenic distillation process.
Furthermore, Abdelhamid has reported the general synthesis methods such as in-situ approach, ex-situ approach, and innovative methods for ZIFs-based membranes. The in-situ approach utilizes modified and unmodified supports for ZIF crystal growth. The ex-situ approach consists of physical methods (rubbing and electrospinning), and chemical methods (dip coating, slip coating, and microwave assisted). The modern and innovative methods to synthesize ZIF-based membrane are contra diffusion, rapid thermal diffusion, electrospray deposition, and 3D printing [21][13].
4. Mechanism of CO2 Capture
The mechanism of CO2 capture and storage by ZIF mainly depends on the thermodynamic and physiochemical properties of the adsorbent. The CO2 storage at high pressure is because of the adsorbate-adsorbate interactions, whereas selective CO2 capture at low pressure is due to the adsorbent-adsorbate interactions. In addition, the adsorbate should have a high contact area and strong polarization interactions. The pores’ design and size have played a significant role, and pores should be large for good interactions in the CO2 capture process. In addition, the adsorbent should have a large volume and surface areas of pores for high CO2 adsorption. Furthermore, the adsorbent should display fundamental properties such as high mechanical stability, high thermal stability, high chemical stability, low heat capacity, high adsorption capacity, low cost, etc. [24][16]. The physical and chemical adsorbents are used for CO2 capture. Zeolite, MOFs, ZIFs, etc., are promising physical adsorbent materials for CO2 capture, whereas amine-based and aqueous diamine are chemical adsorbent materials for CO2 capture.
The mechanism of the CO2 capture process by solvent technology, solid sorbent technology, membrane technology, and cryogenic distillation are discussed further in this section. The charge, charge dispersion, size, intermolecular hydrogen bonds, degree of solvation, etc., play a significant role in the solvent CO2 capture process. In this method, the chemical and physical solvents are used according to the characteristic of the gas stream. Examples of physical solvents are glycol ether and methanol, whereas chemical solvents are alkali carbonates, alkanolamines, and aqueous ammonia [25][17]. In this process, with the help of preferential dissolution, the CO2 is removed from the multi-component gas stream. The flue gases are cooled down at 40–60 °C by lean CO2 solvent. The CO2-rich solvent regenerates steam at a high temperature of 100–140 °C. The CO2 solubility and the sorption rate are the most significant factors during CO2 extraction. In addition, the CO2 solubility depends on the partial pressure of CO2, operating temperature, solvent concentration, type of solvent, and concentration of other components.
In solid sorbent technology, the CO2 separation is achieved by using selective adsorption at the surface of the adsorbent or inside its pore structure. The CO2 sorbent materials should have all the relevant sorption properties such as selectivity, working capacity, heat capacity, sorption rate, stability, etc. For removing CO2, aluminosilicate zeolite, titano-silicate, and activated carbons are the most prevalent porous solids. MOFs, ZIFs, and porous silica are other solid porous candidates for extracting CO2. In this process, adsorption can be accomplished in sorption columns with sorbent materials (particles, pellets, or fluidized bed reactors). The sorption columns are operated by pressure and temperature swing adsorption. In pressure swing adsorption, the adsorption and desorption are triggered by an increase or decrease in the pressure. Likewise, in temperature swing adsorption, the adsorption is carried out at atmospheric pressure, and desorption is activated by raising the temperature. The range for adsorption temperature is 55–60 °C and for desorption is 55–150 °C [26][18].
The currently accessible and functional technology (aqueous amine absorbents) for CO2 capture incudes 30% energy penalty over power generation. This penalty can be reduced by using solid sorbents. The CO2 adsorption can be divided as physical, chemical or both adsorptions. The families of solid sorbents are shown in Figure 4 [27][19]. Several studies have been conducted on solid absorbents for CO2 capture which are based on characteristics of solid absorbents such as selectivity, adsorption capacity, adsorption-desorption kinetics, mechanical properties, chemical and thermal stability, durability, energy consumption of regeneration, etc.
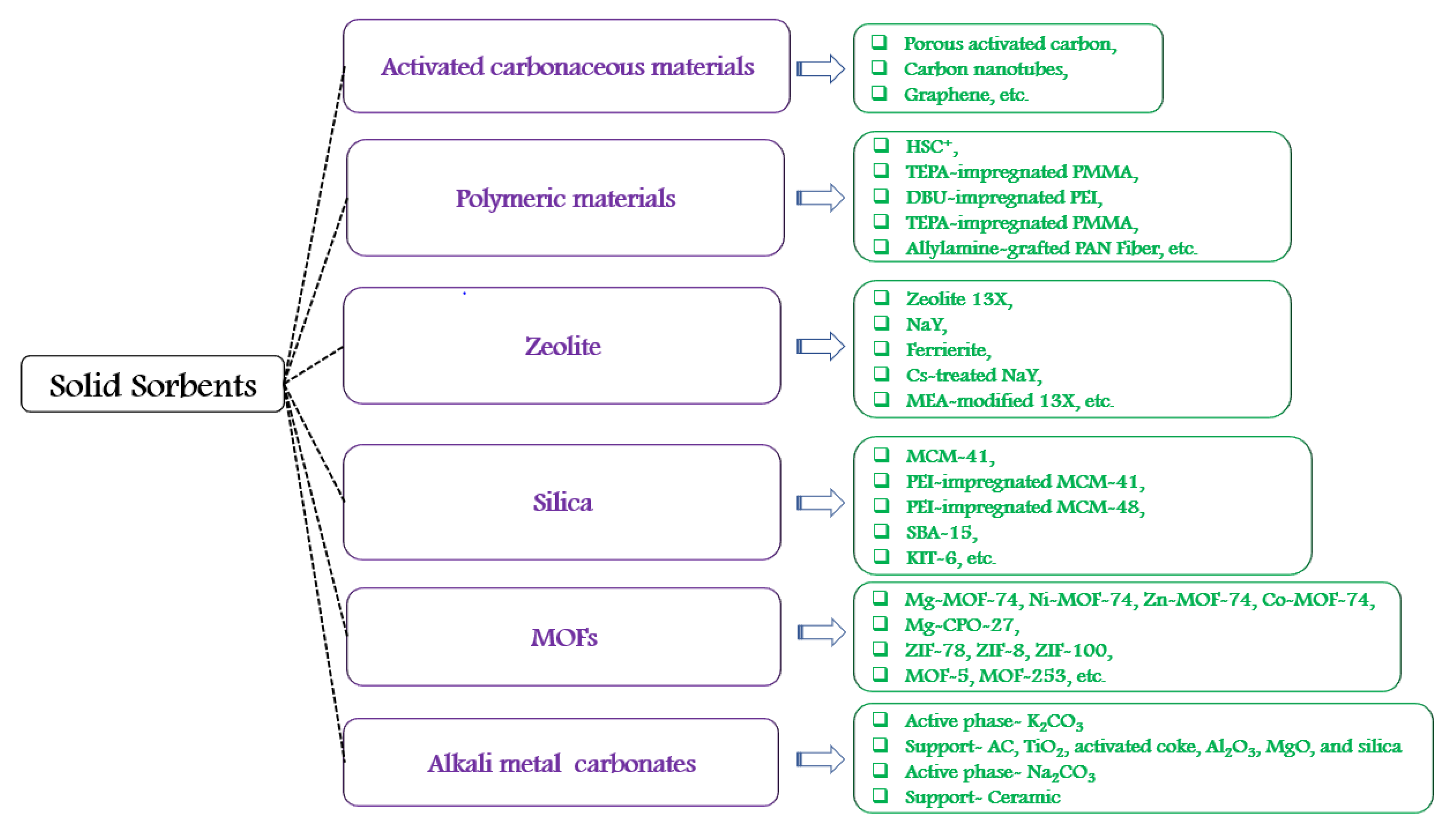
Figure 4.
The families of solid sorbents.
Wang et al. have reviewed recent advancement and trend in solid sorbents for CO2 capture. The review focused on low temperature (less than 200 °C), intermediate temperature (200–400 °C), and high temperature (greater than 400 °C) CO2 sorbents. In addition, the review paper included research of CO2 sorbents from waste resources (nut shells, residues from wood and food, eggshells, fishbones, etc.) [26][18]. Similarly other review paper has investigated the porous support materials such as MCM-41, SBA-15, KIT-6, PMMA, and PS for CO2 adsorption. The review has reported that the development of the solid amine sorbents is very less, and significant research work has to be needed for CO2 adsorption [28][20]. Likewise, Dunstan et al. have reviewed the fundamental aspects of solid sorbents based on alkali and alkaline earth metal oxide for CO2 capture [29][21]. Samanta et al. have reviewed the usage of solid sorbents for post-combustion CO2 capture. This review has carried out comparison and comprehensive study on recent progress, techno-economic analysis, and design aspect of solid sorbents [30][22]. Dao et al. have been studied the CO2 adsorption/desorption enhancement properties of solid sorbents with Tetraethylenepentaammine/Diethanolamine blends. It has been observed from the study that these solid sorbents have high selectivity and cyclic stability [31][23]. Dinda et al. have studied zeolite based solid sorbents using monoethanolamine, ethylenediamine, diethylenetriamine, and triethylenetetramine for CO2 adsorption. The results have shown that these solid absorbents can be used several times without compromising the properties [32][24]. Fu et al. have studied the MgO modified MCM-41 solid sorbents for CO2 capture. The results have concluded that MgO modified MCM-41 has possessed good thermal stability and displayed good CO2 adsorption/desorption properties [33][25]. Yamada et al. have developed amine-impregnated solid sorbents for CO2 adsorption. The results confirmed the high performance of solid sorbents with respect to adsorption, desorption, and regeneration energy [34][26].
Liu et al. have studied the modified MCM-41 impregnated with zeolite A absorbent. The results have confirmed that the adsorption capability of modified sorbent has increased drastically due to the addition of zeolite A [35][27]. The synthesis and characterization of pore expanded MCM-41 have been carried out to determine CO2 adsorption. The results have confirmed high CO2 uptake due to pore expansion [36][28]. Similarly, Guo et al. have investigated the mechanism, kinetics, and performance of mesoporous silica Fe-MCM-41-A for CO2 adsorption [37][29]. Xu et al. developed nanoporous solid sorbent (polyethyleneimine-modified mesoporous molecular sieve of MCM-41 for CO2 capture. It was reported that the loading of polyethyleneimine into MCM-41 has significantly increased the adsorption capacity [38][30]. Cao et al. have evaluated the CO2 adsorption of activated carbon from tire char, chicken waste, commercial activated carbon, and zeolite. The characteristic results have shown that the zeolite has shown the best CO2 adsorption capacity in comparison to other counterparts [39][31]. Likewise, the synthesis of porous solid sorbents (activated carbon and MCM-41 from bagasse and rice husk) has been carried out to study the CO2 adsorption capacity. The results confirmed that activated carbon synthesized from bagasse has shown highest CO2 adsorption capacity in comparison to other sorbents [40][32].
In membrane technology, the CO2 separation is accomplished from flue gas by porous or dense membranes. In a porous membrane, the gas is separated due to the differences in gas diffusion, whereas the differences in reactivity are responsible for gas separation in the dense membrane. In a dense membrane, a solution-diffusion transport mechanism is used to accomplish the gas separation. In this mechanism, the gas molecules initially dissolve into one phase of the membrane and then diffuse across the thickness of the membrane. Membranes are of materials such as polymers, metals, ceramics, hybrids, etc. Polymeric membranes are inexpensive, whereas inorganic materials provide good thermal stability and resist pressure, fouling and chemically aggressive gas steam [41][33]. The flue gas is dried, cooled, and compressed in cryogenic distillation to capture CO2. The flue gas is cooled to a temperature somewhat above the point where CO2 forms a solid. Further cooling results in precipitates of CO2 as a solid, which depends upon the final temperature. At the end of the expansion process, the efficiency of CO2 capture depends on the pressure and temperature. In this process, the steps are O2 fired combustion, which separates O2 from N2, combustion process with pure O2, which produces CO2 and H2O, maintaining temperature and heat loads in the boiler by recirculating some fraction of CO2, condensing H2O to produce pure CO2, then the CO2 compression and CO2 separation process [42][34].
5. ZIF Characterization
Several tools are used to study the CO2 capture mechanism and characterize the structural response. The surface area measurements and scanning electron micrograph are used to investigate ZIF’s textural and morphological characteristics for CO2 capture. N2-CO2 adsorption-desorption isotherms are used to characterize textural properties of surface area/pore volume of ZIFs. Furthermore, X-ray diffraction (X-ray absorption of fine structure) has been used to examine the structural activity relationship in ZIFs. Additionally, the X-ray absorption near edge spectroscope and the extended X-ray absorption fine structure can provide oxidation state, charge transfer, bond distance, coordination number, etc. Additionally, TGA investigates the structural or thermal stability of ZIFs. The characterization results are used to compare ZIFs based on crystal structure, pore size, pore geometry, crystallite size, surface area, particle morphology, chemical compositions, particle size distributions, adsorption sites, CO2 capture capacity, CO2 sorption rate, CO2 selectivity, thermal stability, chemical stability, mechanical stability, regeneration ability, etc.References
- Tan, J.C.; Bennett, T.D.; Cheetham, A.K. Chemical structure, network topology, and porosity effects on the mechanical properties of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2010, 107, 9938–9943.
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Accounts Chem. Res. 2010, 43, 58–67.
- Yan, S.; Zhu, D.; Zhang, Z.; Li, H.; Chen, G.; Liu, B. A pilot-scale experimental study on CO2 capture using Zeolitic imidazolate framework-8 slurry under normal pressure. Appl. Energy 2019, 248, 104–114.
- Song, F.; Cao, Y.; Zhao, Y.; Jiang, R.; Xu, Q.; Yan, J.; Zhong, Q. Ion-Exchanged ZIF-67 Synthesized by One-Step Method for Enhancement of CO2 Adsorption. J. Nanomater. 2020, 2020, e1508574.
- Morris, W.; Leung, B.; Furukawa, H.; Yaghi, O.K.; He, N.; Hayashi, H.; Houndonougbo, Y.; Asta, M.; Laird, B.B.; Yaghi, O.M. A Combined Experimental–Computational Investigation of Carbon Dioxide Capture in a Series of Isoreticular Zeolitic Imidazolate Frameworks. J. Am. Chem. Soc. 2010, 132, 11006–11008.
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319, 939–943.
- Bhattacharjee, S.; Jang, M.-S.; Kwon, H.-J.; Ahn, W.-S. Zeolitic Imidazolate Frameworks: Synthesis, Functionalization, and Catalytic/Adsorption Applications. Catal. Surv. Asia 2014, 18, 101–127.
- Kukkar, P.; Kim, K.-H.; Kukkar, D.; Singh, P. Recent advances in the synthesis techniques for zeolitic imidazolate frameworks and their sensing applications. Coord. Chem. Rev. 2021, 446, 214109.
- Lee, W.-C.; Chien, H.-T.; Lo, Y.; Hao Che, C.; Wang, T.; Kang, D.-Y. Synthesis of Zeolitic Imidazolate Framework Core–Shell Nanosheets Using Zinc-Imidazole Pseudopolymorphs. ACS Appl. Mater. Interfaces 2015, 7, 18353–18361.
- Qian, X.; Ren, Q.; Wu, X.; Sun, J.; Wu, H.; Lei, J. Enhanced Water Stability in Zn-Doped Zeolitic Imidazolate Framework-67 (ZIF-67) for CO2 Capture Applications. ChemistrySelect 2018, 3, 657–661.
- Wang, Q.; Xia, W.; Guo, W.; An, L.; Xia, D.; Zou, R. Functional Zeolitic-Imidazolate-Framework-Templated Porous Carbon Materials for CO2 Capture and Enhanced Capacitors. Chem. Asian J. 2013, 8, 1879–1885.
- Wu, T.; Dong, J.; De France, K.; Zhang, P.; Zhao, X.; Zhang, Q. Porous carbon frameworks with high CO2 capture capacity derived from hierarchical polyimide/zeolitic imidazolate frameworks composite aerogels. Chem. Eng. J. 2020, 395, 124927.
- Abdelhamid, H. A Review on Removal of Carbon Dioxide (CO2) using Zeolitic Imidazolate Frameworks: Adsorption and Conversion via Catalysis. Catalysis 2022.
- Sr, V.; Ma, C. Highly Permeable Zeolite Imidazolate Framework-8 Membranes for CO2/CH4 Separation. J. Am. Chem. Soc. 2010, 132, 76–78.
- Railey, P.; Song, Y.; Liu, T.; Li, Y. Metal organic frameworks with immobilized nanoparticles: Synthesis and applications in photocatalytic hydrogen generation and energy storage. Mater. Res. Bull. 2017, 96, 385–394.
- Pourhakkak, P.; Taghizadeh, M.; Taghizadeh, A.; Ghaedi, M. Chapter 2—Adsorbent. In Interface Science and Technology; Ghaedi, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 71–210.
- Fazari, F. Carbon Dioxide Capture by Chemical Absorption: A Solvent Comparison Study; Massachusetts Institute of Technology: Cambridge, MA, USA, 2010.
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518.
- Shi, Y.; Liu, Q.; He, Y. CO2 Capture Using Solid Sorbents. In Handbook of Climate Change Mitigation and Adaptation; Chen, W.-Y., Suzuki, T., Lackner, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 2349–2404.
- Ünveren, E.E.; Monkul, B.; Sarıoğlan, Ş.; Karademir, N.; Alper, E. Solid amine sorbents for CO2 capture by chemical adsorption: A review. Petroleum 2017, 3, 37–50.
- Dunstan, M.; Donat, F.; Bork, A.H.; Grey, C.; Müller, C. CO2 capture using solid sorbents: Fundamental aspects, mechanistic insights and recent advances. Chem. Rev. 2021, 121, 12681–12745.
- Samanta, A.; Zhao, A.; Shimizu, G.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2011, 51, 1438–1463.
- Dao, D.S.; Yamada, H.; Yogo, K. Enhancement of CO2 Adsorption/Desorption Properties of Solid Sorbents Using Tetraethylenepentamine/Diethanolamine Blends. ACS Omega 2020, 5, 23533–23541.
- Dinda, S.; Murge, P.C.; Paruchuri, B.C. A study on zeolite-based adsorbents for $$\hbox CO2 capture. Bull. Mater. Sci. 2019, 42, 240.
- Fu, X.; Zhao, N.; Li, J.; Xiao, F.; Sun, Y. Carbon Dioxide Capture by MgO-modified MCM-41 Materials. Adsorpt. Sci. Technol. 2009, 27, 593–601.
- Yamada, H.; Dao, D.; Chowdhury, F.; Fujiki, J.; Goto, K.; Yogo, K. Development of Amine-impregnated Solid Sorbents for CO2 capture. Energy Procedia 2014, 63, 2346–2350.
- Liu, M.; Hou, L.; Yu, S.; Xi, B.; Zhao, Y.; Xia, X. MCM-41 impregnated with A zeolite precursor: Synthesis, characterization and tetracycline antibiotics removal from aqueous solution. Chem. Eng. J. 2013, 223, 678–687.
- Loganathan, S.; Tikmani, M.; Ghoshal, A.K. Novel Pore-Expanded MCM-41 for CO2 Capture: Synthesis and Characterization. Langmuir ACS J. Surf. Colloids 2013, 29, 3491–3499.
- Guo, Y.; Chen, B.; Zhao, Y.; Yang, T. Fabrication of the magnetic mesoporous silica Fe-MCM-41-A as efficient adsorbent: Performance, kinetics and mechanism. Sci. Rep. 2021, 11, 26.
- Xu, X.; Song, C.; Andresen, J.; Miller, B.; Scaroni, A. Novel Polyethylenimine-Modified Mesoporous Molecular Sieve of MCM-41 Type as High-Capacity Adsorbent for CO2 Capture. Energy Fuels 2001, 16, 1463–1469.
- Zhao, H.-Y.; Cao, Y.; Lineberry, Q.; Pan, W.-P. Evaluation of CO2 adsorption capacity of solid sorbents. J. Therm. Anal. Calorim. 2011, 106, 199–205.
- Boonpoke, A.; Chiarakorn, S.; Laosiripojana, N.; Towprayoon, S.; Chidthaisong, A. Synthesis of Activated Carbon and MCM-41 from Bagasse and Rice Husk and their Carbon Dioxide Adsorption Capacity. 2. J. Sustain. Energy Environ. 2010, 2, 77–81.
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125.
- Abu-Zahra, M.R.M.; Sodiq, A.; Feron, P.H.M. 29—Commercial liquid absorbent-based PCC processes. In Absorption-Based Post-Combustion Capture of Carbon Dioxide; Feron, P.H.M., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 757–778.
More
