Hydroxycinnamic acids (HCAs) are important natural phenolic compounds present in high concentrations in our food products. Dietary intake and nutritional importance of HCAs is briefly described along with their pharmacokinetic properties, which have a high impact on HCAs to reach the target tissue in order to exert their biological activities. A range of health beneficial effects were observed for HCAs and in recent years, also for their metabolites formed in gastrointestinal tract, liver and kidneys. Therefore, metabolism is of high importance since HCAs’ metabolites could retain, enhance or lose the biological activity of corresponding parental HCAs. The biological activities and health benefits of HCAs' metabolites are also briefly reviewed.
- diet
- natural compounds
- phenolic acids
- hydroxycinnamic acids
- metabolites
- pharmacokinetic properties
- biological activities
- health effects
1. Definition
Our diet rich in plant food contains several health-beneficial ingredients. Among such ingredients, polyphenols represent one of the most important natural compounds. Phenolic compounds are members of probably the largest group of plant secondary metabolites and have the main function to protect the plants against ultraviolet radiation or invasion by pathogens[1][2]. They can be divided into four distinct classes based on the number of phenol rings and structural fragments connecting them, namely phenolic acids, flavonoids, stilbenes and lignans[2]. The first class generally involves the phenolic compounds possessing a carboxylic acid as the main functional group[3], thus being named as phenolic acids, which are further split into two groups, namely hydroxybenzoic and hydroxycinnamic acids (HCAs) (Figure 1).
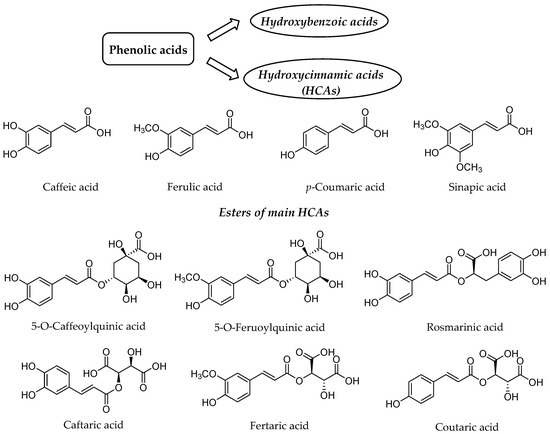
Figure 1. Structure of the main hydroxycinnamic acids (HCAs) and their esters as one of the major class of phenolic acids.
2. Introduction
HCAs possess phenylpropanoid C6-C3 structure as the main chemical scaffold and are recognized by the presence of hydroxyl group(s) on the aromatic ring(s) and a carboxyl group in the lateral chain[4][5]. The number and position of hydroxyl groups and other substituents contribute to the diversity of HCAs. The most abundant HCAs in nature are para-coumaric, caffeic, ferulic, and sinapic acids (Figure 1)[5][6]. In nature, all four acids are rarely present in a free form and are usually esterified with quinic and tartaric acids or various derivatives of carbohydrates[7]. Chlorogenic acids are one the most abundant esters including the whole set of HCAs esters with quinic acid, namely caffeoyl-, feruloyl-, dicaffeoyl- and coumaroylquinic acids[8][9]. The most common representative is 5-O-caffeoylquinic acid (Figure 1) often referred to as chlorogenic acid[9]. An ester of caffeic acid and 3,4-dihydroxyphenyllactic acid is called rosmarinic acid (Figure 1), which is one of the most abundant caffeic acid ester in the plant kingdom besides chlorogenic acids[10].
Caffeic acid presents up to 70% of whole HCAs in fruits, whereas ferulic acid is the prevalent HCA in cereal grains[7]. The daily consumption of HCAs varies significantly between individuals[11][12][13], which is attributed not only to different intake but also diverse metabolism and absorption from the gut. The bioavailability and metabolism of HCAs and their conjugates is thus of high importance for health benefits for particular individual.
Herein, we will briefly present natural sources and pharmacokinetic properties of HCAs and their esters. Afterwards, the main focus will be on their metabolism, biological activities and health benefits with emphasis on specific effects of HCAs mediated by their metabolites.
3. Dietary Intake and Nutritional Importance of HCAs
HCAs are one of the most widely distributed naturally occurring phenolic acids being typically present in the form of esters with quinic, shikimic or tartaric acid, saccharides, flavonoids or with plant structural elements (i.e., cellulose, lignin and proteins)[9][14][15]. HCAs are considered as important constituents of our diet, contributing to taste, color, nutritional value and health benefits[11]. HCAs are thus present at a wide concentration range in our everyday food and drinks, including fruits (apples, berries, plums, cherries, peaches and some citrus fruits), vegetables (carrots, salad, cabbage, eggplant, and artichoke), cereals, beverages (tea, coffee), grapes and wine[11][16][17][18][19]. HCA derivatives represent about 18% of all phenolic compounds in apples with chlorogenic acid as the most abundant HCA in the entire apple (up to 87% of the total HCA amount)[20], whereas p-coumaric, caffeic and ferulic acids are encountered in blueberry fruits[19]. Indeed, caffeic acid is the most abundant in fruits (between 75 and 100% of the total HCA content) with the highest quantities in the range of 0.5 to 2 g in blueberries, kiwis, plums, cherries, and apples, whereas ferulic acid is ubiquitous in cereal grains, which represent its major dietary source[21]. For example, ferulic acid is the prevalent phenolic acid in barley brans and seeds[19] and is also present in blueberries and blackberries ranging from 2.99 to 16.97 mg/g fresh weight[22]. Similarly, the levels of p-coumaric and caffeic acids in blueberry fruits varies from 0.40 to 15.78 and 1.38 to 6.32 mg/g fresh weight, respectively[22]. The most abundant HCAs in cranberry fruit are p-coumaric and sinapic acids with approximately 0.25 and 0.21 g/kg fresh weight, respectively[23]. All major HCAs are also present in numerous vegetables, with an average amount of total phenolic acids up to 32.0 mg/100 g fresh weight[24]. The major soluble HCAs identified in breeding vegetables are chlorogenic acids (eggplant, carrot, basil, spinach, Chinese cabbage, parsnip, lettuce, pepper, cauliflower, turnip, green bean, tomato), p-coumaric acid (radish, pepper, cauliflower, white cabbage, onion, zucchini, cucumber), ferulic acid (red beet, radish, pepper, turnip, cucumber), caffeic acid (carrot, broccoli, zuccini) and sinapic acid (broccoli, Chinese cabbage, cauliflower, turnip, white cabbage, pea)[24]. In another study, ferulic acid and caffeic acid were identified at high concentrations in spinach (18.0–41.4 mg/kg dry weight) and garlic (1.7–28.3 mg/kg dry weight), respectively, while chlorogenic acid was determined as the most abundant HCAs in artichoke (37.8–734.7 mg/kg dry weight)[25]. Chlorogenic acids are also one of the main constituents of green coffee beans[26], with daily intake in the range from 120 to 594 mg for regular coffee drinkers[27], whereas caffeoyl- and p-coumaroyl-quinic acids were identified in tea leaves[28]. Indeed, coffee beans are one of the richest sources of chlorogenic acids in the diet, leading to highly variable levels in coffee brews according to the literature data[27]; however, typical values of chlorogenic acids and their main lactones are in the range from 50 to 200 mg/mL. Ferulic, p-coumaric and caffeic acid are located in skin’s vacuoles and pulp cells of grapes being esterified with tartaric acid and named as fertaric, p-coutaric and caftaric acids (Figure 1), respectively[29]. Caftaric acid thus presents an important phenolic compound in white (6–73 mg/L) or red wine (46–141 mg/L)[11][16][30]. HCAs can be also found in mushrooms. Small amounts of caffeic, ferulic and p-coumaric acids were determined in the extracts from Polish wild growing edible mushrooms with the exception of Pholiota mutabilis, which contained 29.10 mg/kg dry weight of p-coumaric acid[31].
HCAs are generally ingested daily in high amounts, which vary significantly between individuals—the estimated intake of 46.3 to 78.9 mg/day for children and 153.6 to 231.8 for adults was determined in cross-sectional analysis of UK National Diet and Nutrition Survey Rolling Programme[12]. Another study estimated the average phenolic acid consumption for men and women of 222 mg per day, dominated by caffeic acid with 206 mg of daily intake[13]. The prevailing dietary sources of HCAs are coffee and fruits with 92% of the caffeic acid and 59% of the p-coumaric acid intake, respectively[13].
4. Bioavailability of HCAs
From the nutritional point of view, bioavailability is described as the fraction of a given food which our body can utilize and it is highly affected by various factors, such as bioaccessibility, the food matrix effect, transporters, molecular structures, metabolizing enzymes and absorption[32].
4.1. Food Processing and Bioaccessibility
In order to exert a range of health beneficial effects after the consumption of plant-derived food, bioactive phytochemicals need to withstand food processing and release from the food matrix after ingestion[32][33]. Furthermore, after release in the gastrointestinal tract (GIT) (bioaccessibility), the uptake of the active compounds along with metabolism in the GIT and liver is also highly important to reach the target tissue responsible for their biological activity.
The bioavailability of phenolic acids rely upon their form (free or conjugated) present in the food matrix; thus, it could also be affected by food processing[11]. The main example of high food processing influence is cereals, where the majority of the edible fiber-bound phenolic acids are esterified to the cell walls and are common components of complex structures (such as hydrolysable tannins, lignins, organic acids), thus being poorly bioavailable[11][34]. For example, ferulic acid as a dominant HCA in oats is bound to the cell wall arabinoxylan or dimerized through oxidative cross-linking[34]. Many conventional processing techniques (i.e., cleaning and heat treatment, dehulling and cutting, flaking or milling, germination) remove HCAs or increase the levels of free acids in oat and cereal food products[34]. Therefore, an optimized processing has a noteworthy influence on the absorption of bioactive compounds such as HCAs, being especially important in case of tightly-bound ferulic acid[35].
The second important factor affecting the bioavailability of HCAs after appropriate food processing is the release from the food matrix after ingestion, which can be defined by the term bioaccessibility. Bioaccessibility is affected by the composition of the consumed food matrix and physicochemical properties (e.g., pH, temperature and the texture of the matrix)[32]. A considerable percentage of HCAs exhibit low bioaccessibility because of the structural complexity of the plant’s cell wall[36]. The investigation of the effects of boiling and extrusion processes applied in sorghum bran, a known source of HCAs, showed the improvement of the HCAs’ release and rise of the antioxidant capacity. In case of ferulic acid from boiled or extruded sorghum bran, higher bioaccessibility in the GIT was observed. Therefore, it was showed that food matrix and in vitro digestion conditions along with applied technological processes have an important impact on the release of HCAs[36]. Furthermore, the interaction with digestive enzymes could also alter the bioaccessibility of HCAs.
4.2. Absorption, Distribution, Metabolism and Excretion of HCAs
The rate and extent of the absorption of HCAs from the GIT to the systemic circulation generally depends on their structure[37]. It is known that the presence of an ester moiety results in lower HCAs absorption[38]. Several studies demonstrated that bound HCAs (for instance ferulic and caffeic acid esters) have reduced absorption capacity through enterocytes in the gastrointestinal wall compared to their free forms[38][39]. On the other hand, HCAs in a free form are rapidly absorbed throughout the GIT, whereas HCA esters or HCAs attached to cell walls are hydrolyzed by esterases before absorption[38]. According to in situ or ex vivo absorption models, ferulic, caffeic and p-coumaric acids could be absorbed from the stomach, jejunum, ileum and colon based on the studies in rats, which were summarized in a brief review by Zhao and Moghadasian[40]. In case of ferulic acid, the colon represents the key site of absorption due to the presence of microbial cinnamoyl esterases, which facilitate its release from the food matrix or parent compounds[41][42]. In another study using the in vitro model for the colonic epithelium (Caco-2/HT29-MTX co-culture cell model), it was suggested that ferulic acid is absorbed via two distinct mechanisms, i.e., passive transcellular diffusion and facilitated transport[42]. Furthermore, the active absorption via the monocarboxylic acid transporter was proposed for some HCAs (i.e., ferulic acid[43], p-coumaric acid[44]) in Caco-2 cells. On the other hand, caffeic acid has low affinity for this transporter and is generally more efficiently absorbed via paracellular pathways, i.e., paracellular diffusion[45]. It was shown that the absorption efficiency of HCAs in vivo is increased in the order from rosmarinic acid, caffeic acid to p-coumaric acid[46]. The absorption of caffeic acid has also been investigated in many other studies[39][47][48][49]. The in situ vascularly perfused rat intestinal preparation, which enables precise and indirect assessment of the contribution of intestinal absorption, was employed to determine the extent of caffeic acid absorption of 12.4% after intraduodenal administration. Furthermore, poor permeability across the Caco-2 cell monolayer was shown for caffeic acid[49]. Thus, it was proposed that the poor bioavailability of caffeic acid in rats (determined as 14.7% in this study) is connected to low absorption from the GIT along with low permeability across the Caco-2 cell monolayer.
One of the most abundant sources of caffeic acid in nature is 5-O-caffeoylquinic acid, which is most likely hydrolyzed to caffeic and quinic acids by esterases from colonic microflora[50] and are not degraded and absorbed in the upper GIT[41]. However, the study from Olthof and coauthors showed that about 33% of chlorogenic acid and 95% of caffeic acid is absorbed in the small intestine of humans[47]. The increased hydrophilic characteristics of the quinic moiety in ester most likely have an impact on the rate and extent of absorption and are responsible for changing its permeability across the epithelium[38]. Thus, part of ingested 5-O-caffeoylquinic acid will reach the blood circulation, while most of it will proceed to the colon and hydrolyse to caffeic and quinic acids. On the other hand, p-coumaric acid exhibits higher bioavailability compared to chlorogenic, caffeic and ferulic acids, being absorbed in rats throughout the whole GIT (including stomach, jejunum, ileum and colon) having the highest absorption rate in jejunum[40][51]. While p-coumaric acid in a free form is easily and quickly absorbed in the upper GIT, its conjugates exhibit much fewer and slower absorption, with higher proportion reaching the colon[51].
The distribution of HCAs within the body along with high absorption has a really important influence on health-beneficial effects of HCAs[46]; however, there have not been many studies conducted about the distribution of HCA to target tissues. In a pharmacokinetic study of caffeic acid from the methanol seed extract of S. cumini in rats, its disposition from the plasma to more perfused tissues was observed in one hour after absorption[52]. Even though caffeic acid was rapidly absorbed from the GIT of rats, only small amounts (19.1%) of ingested dose reached the circulatory system. According to the data obtained about clearance (21.86h) and volume of distribution (4.378), a good safety profile for caffeic acid due to a small time of exposure was proposed[52]. Another study in ddY mice demonstrated distribution of caffeic acid in the plasma, liver and skin following oral administration, absorption and metabolism into conjugated and/or methylated derivatives[53]. It was showed that caffeic acid is efficiently transported in the skin, being able to prevent the damage by UVA-induced generation of reactive oxygen species. The pharmacokinetic study of ferulic acid as the main metabolite of angoroside C in rats showed that ferulic acid is also distributed in some major organs, namely liver, lung, spleen and kidney, with the highest concentration detected after 6 hours, especially in kidneys[54]. In order to follow the distribution of polyphenolic compounds including major HCAs (caffeic, ferulic, sinapic and o-, m- and p-coumaric acids) to target tissues in rats the intravenous administration of 23 polyphenol microbial metabolites was performed by Gasperotti et al.[55]. The kinetics of distribution of the aforementioned HCAs and metabolites in the blood, brain, heart, liver, kidney, and urine showed their accumulation in the kidneys. Due to their low concentrations in liver, it can be concluded that absorbed HCAs’ metabolites from the colon are subjected to limited first-pass metabolism leading to rapid distribution to the other organs after absorption[55]. The pharmacokinetic study of 5-O-caffeoylquinic acid in rats demonstrated distribution to the highly perfused tissues (e.g., liver, kidneys), indicating the importance of an organ’s blood flow and perfusion rate for distribution process of 5-O-caffeoylquinic acid[56]. It was also found in the lung, heart, and spleen; however, the highest amounts were present in liver. Following the levels of 5-O-caffeoylquinic acid in organs indicated its rapid metabolism since it could not be detected there anymore after 4h[56]. In another study in rats, 5-O-caffeoylquinic acid was quickly absorbed after its intranasal administration and high levels in cerebrospinal fluid of rat brain was observed indicating direct nose-to-brain distribution of 5-O-caffeoylquinic acid, which implies the potential use in the therapy of neurodegenerative disorders[57].
The uptake and distribution of HCAs is also highly dependent on their metabolism, which can occur in the GIT, liver and kidneys (Figure 2)[58][59]. Indeed, first-pass metabolism has an important impact on the bioavailability, and consequently also on the bioefficacy of HCAs. Following the ingestion and absorption, HCAs are conjugated by glucuronidation, methylation, and sulfation reactions, which are catalyzed and regulated by specific enzymes (Figure 2)[59]. The conjugation of the hydroxyl group(s) of phenolic compounds, which is/are present in HCAs, can occur with glucuronate or sulfate[60]. The glucuronidation and sulfation of HCAs in the GIT and liver are catalyzed by uridine-5′-diphosphate-glucuronosyltransferases (UGTs) and sulfotransferases (SULTs)[61]. Furthermore, O-methylation also occurs and is catalyzed by catechol-O-methyltransferases (COMTs). HCA esters (e.g., chlorogenic acids) are hydrolyzed by esterases[62]. According to in vitro studies, chlorogenic acids display different susceptibility to hydrolysis, which can occur in the stomach or upper GIT, with 5-caffeoylquinic acid being hydrolyzed more readily by intestinal chlorogenate esterase compared to 3- and 4-caffeoylquinic acid[63]. Glucuronidation, sulfation, methylation and also hydrogenation can take place in enterocytes and liver, whereas conjugation with glycine is acknowledged to only kidneys and liver. In the latter demethylation and dehydrogenation also occurs[64]. The intestinal metabolism is also highly affected by gut microbiota (Figure 2). The human GIT microbiota transform the ingested HCAs to metabolites that usually show higher activity and better absorption compared to the parent compounds. Gut microbiota metabolic transformations can be divided into three main reactions: hydrolysis (O-deglycosylations, hydrolysis of esters), cleavage (C-ring cleavage, demethylation) and reductions (dehydroxylation and hydrogenation) (Figure 2)[58].
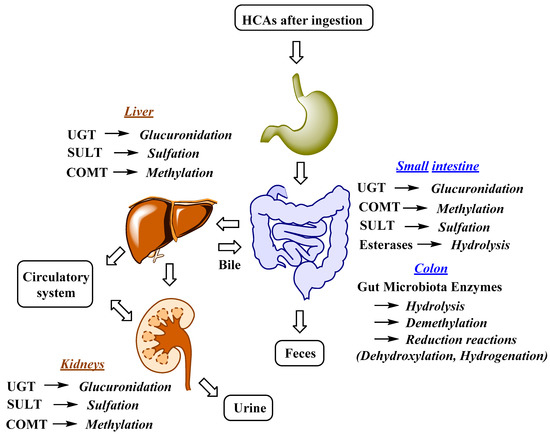
Figure 2. Major metabolic reactions, enzymes and organs involved in HCAs metabolism; UGT, uridine-5′-diphosphate-glucuronosyltransferase; COMT, catechol-O-methyltransferase; SULT, sulfotransferase[58][59].
Enterocyte-like differentiated Caco-2 cells are one of the most utilized in vitro models for examination of small intestinal epithelium metabolism[65]. The investigation of the metabolism of the main HCAs and their esters in vitro in the Caco-2 model demonstrated glucuronidation, methylation, and sulfation of free and methyl-HCAs along with hydrolysis, which occurred extra- and intracellularly. According to the results obtained in this study, sulfation could be the preferential metabolic reaction for HCAs in the epithelium of small intestine[65]. In case of ferulic acid, which is efficiently transported over the intestinal barrier in a free form, only low amounts of conjugates (feruloyl-glucuronide or sulfate, as well as some free dihydroferulic acid) with feruloyl-glucuronide as a main metabolite was observed in the in vitro model for human small intestinal epithelium (Caco-2/HT29-MTX co-culture cell model)[42]. The proposed main metabolic pathways and metabolites of 5-O-caffeoylquinic, caffeic and ferulic acids are presented in Figure 3[48][66][67].
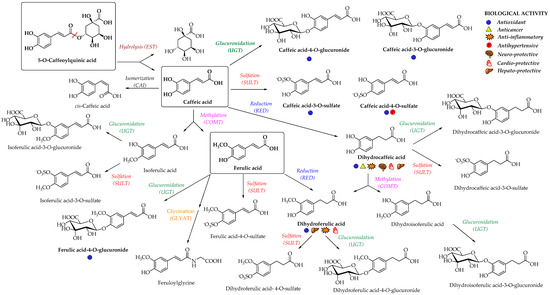
Figure 3. Proposed metabolic pathways and metabolites of 5-O-caffeoylquinic, caffeic and ferulic acids; EST, esterase; CAI, caffeic acid isomerase; RED, reductase; COMT, catechol-O-methyltransferase; SULT, sulfotransferase; UGT, uridine-5′-diphosphate-glucuronosyltransferase; GLYAT, glycine-N-acyltransferase[48][66][67]. The main biological activities of selected metabolites are presented with appropriate symbols.
In a study from Stalmach et al.[67], the metabolite profiling of HCA’s derivatives in human plasma and urine following the coffee ingestion was performed. Caffeoyl and feruloylquinic acids are one of the most abundant coffee ingredients; however, only trace levels of 5-caffeoylquinic acid and low levels of three different feruloylquinic acids appeared in the circulatory system. This is due to the presence of intestinal esterases[62], which hydrolyse 5-caffeoylquinic acid to caffeic acid that is further metabolized to caffeic acid-3-O-sulfate (Figure 3)[67]. As already noted, the hydrolysis of the remaining 5-caffeoylquinic acid into caffeic and quinic acids is catalyzed by esterases provided by the gut microbiota[50]. In addition to caffeic acid-3-O-sulfate, ferulic acid-4-O-sulfate (Figure 3) was also detected in plasma, probably as a result of a parallel ferulic acid metabolism involving the hydrolysis of feruloylquinic acids, caffeic acid methylation by COMTs and conversion of caffeoylquinic acids to feruloylquinic acids, thereby contributing to the ferulic acid pool[67].
The hepatic uptake and metabolism of HCAs was also studied using human hepatoma HepG2 cells as a hepatic model system[68]. Moderate uptake of caffeic and ferulic acids was observed, while chlorogenic acid showed null metabolism and very limited absorption. In case of caffeic acid, methylation was found to be the preferential metabolic pathway along with sulfation and glucuronidation, whereas ferulic acid converted to glucuronides as the only metabolites[68]. Another study in the human liver S9 homogenates showed that sulfation compared to glucuronidation is more preferred, being the most efficient and high-affinity pathway for HCAs’ metabolism in liver[61]. The highest efficiency of conjugation was demonstrated in caffeic acid, followed by ferulic, dihydrocaffeic, isoferulic and dihydroferulic acids[38][61]. Similarly, absorbed p-coumaric acid can also undergo the conjugation with glucuronide, sulfate and sulfoglucuronide (diconjugation with sulfate and glucuronic acid) in the liver[51]; however, sulfoglucuronide is more typical for ferulic acid and was showed as the leading metabolite (60–70% of the total) along with ferulic acid glucuronide and sulfate in the rat’s plasma following the administration of free ferulic acid or its sugar esters[69]. The bioavailability study of yerba mate containing phenolic compounds in humans led to the identification of 34 metabolites in biological fluids with sulfates of caffeic, ferulic and isoferulic acids as the prevailing metabolites[70]. The main metabolites determined in plasma as a consequence of delayed colonic absorption after colonic microbiota metabolism were reduced forms of HCAs (i.e., dihydroferulic, dihydrocaffeic and dihydroisoferulic acids) and their phase II conjugates (i.e., dihydrocaffeic acid-4-O-sulfate, dihydroferulic acid-4-O-glucuronide, dihydroisoferulic acid-3-O-glucuronide, dihydroferulic acid-4-O-sulfate and dihydroisoferulic acid-3-O-sulfate) in addition to feruloylglycine[70].
In addition to glucuronidation and/or sulfation, HCAs can also oxidize into benzoic acid derivatives that are further converted into hippuric acid derivatives[11]. For example, the metabolism of chlorogenic acid by GIT microbiota into diverse aromatic acid metabolites (e.g., m-coumaric acid, benzoic and phenylpropionic acids derivatives) was observed[71]. Indeed, the metabolites of microbial origin, such as m-coumaric, 3,4-dihydroxyphenylpropionic, 3-(3-hydroxyphenyl)propionic acid, 3-hydroxybenzoic, 3-hydroxyhippuric and hippuric acids (Figure 4) were detected in plasma and urine after chlorogenic acid diet in rats indicating the high importance of gut microflora metabolism to bioavailability of HCAs[71]. It was suggested that the preferred route of caffeic acid metabolism in rats is to 3-(3-hydroxyfenil)propionic acid, whereas 3-hydroxyhippuric acid is mainly excreted in the urine of humans[72]. Hippuric acid is mainly produced as a result of quinic acid moiety metabolism; however, it can also originate from other metabolites in the caffeic acid metabolic pathway. According to the gastrointestinal model studies, the gut microbiota metabolism of chlorogenic, ferulic and caffeic acids (Figure 4) generates various cinnamic acids (caffeic, ferulic, coumaric, dihydrocaffeic and cinnamic acids), phenyl substituted propionic acids (3-(3,4-dihydroxyphenyl)propionic, 3-phenylpropionic, and 3-(4-hydroxy-3-methoxyphenyl)propionic acids), benzoic acids (vanillic, 3-hydroxybenzoic and benzoic acids) and 3-hydroxyphenylacetic acid[73][74]. In case of p-coumaric acid, the observed plasma metabolites were m-dihydrocoumaric acid and dihydrocoumaric acid-O-sulfate[51].
Due to the fast and considerable metabolism of HCAs, the majority of their metabolites are quickly excreted in bile (largely conjugated metabolites) and urine (small conjugates, e.g., sulfates)[18][75]. There have been many studies in rats and humans about the HCAs’ excretion via the urinary and biliary pathways and they were described or reviewed elsewhere[18][47][48][72][76][77][78][79][80]; thus, we will briefly mention only the recent ones. The recent study of the urinary excretion rates of the main HCAs in non-fasted rats demonstrated the highest excretion rate for ferulic acid, followed by caffeic and p-coumaric acids, with all being absorbed, metabolyzed, and excreted in the urine within 6h, while chlorogenic acids showed much smaller and slower urinary excretion (up to 48h)[81]. Relatively fast urinary excretion (up to 8h) of 30 various metabolites was observed following the ingestion of oat bran in humans. The highest concentrations in the urine were determined for vanillic and hydroxylated hippuric acids (especially at positions 3 and 4), and ferulic acid-4-O-sulfate[82]. Generally, in most studies, up to date HCAs’ derivatives in the form of sulfates, glucuronides and on a smaller scale also glycine conjugates have been usually excreted in the urine[83]. Many other metabolites have also been identified in the urine resulting from metabolism in liver and kidneys or from biotransformations by gut microbiota and are summoned in a recent book by Farah[83].
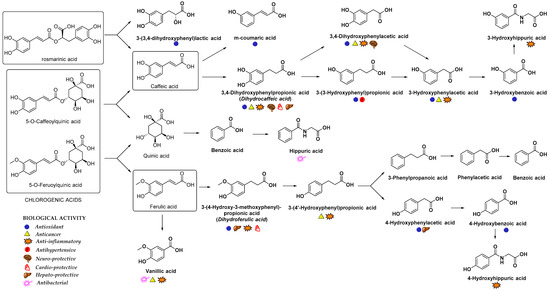 Figure 4. Main metabolites of rosmarinic, chlorogenic, caffeic and ferulic acids that appeared after biotransformation by gut microflora[63][71][84][85]. The main biological activities of selected metabolites are presented with appropriate symbols.
Figure 4. Main metabolites of rosmarinic, chlorogenic, caffeic and ferulic acids that appeared after biotransformation by gut microflora[63][71][84][85]. The main biological activities of selected metabolites are presented with appropriate symbols.
References
- Aldred, E.M.; Buck, C.; Vall, K. Chapter 21-Phenols. In Pharmacology; Aldred, E.M., Buck, C., Vall, K., Eds.; Churchill Livingstone: Edinburgh, UK, 2009; pp. 149–166.
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747.
- Naresh Kumar; Nidhi Goel; Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnology Reports 2019, 24, e00370, 10.1016/j.btre.2019.e00370.
- Teixeira, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic acizd antioxidants: An electrochemical overview. Biomed. Res. Int. 2013, 2013, 251754.
- Vinholes, J.; Silva, M.; Silva, L.R. Hydroxycinnamic acids (HCAS): Structure, biological properties and health effects. In Advances in Medicine and Biology; Leon, V., Berhardt, L.V., Eds.; Nova Biomedical: Waltham, MA, USA, 2015; Volume 88, pp. 105–130.
- Rui Hai Liu; Potential synergy of phytochemicals in cancer prevention: mechanism of action.. The Journal of Nutrition 2004, 134, 3479S-3485S, 10.1093/jn/134.12.3479s.
- Sophie Lafay; Angel Gil-Izquierdo; Bioavailability of phenolic acids. Phytochemistry Reviews 2007, 7, 301-311, 10.1007/s11101-007-9077-x.
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244.
- Clifford, M.N. Chlorogenic acids and other cinnamates–nature, occurrence, dietary burden, absorption and metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043.
- Maike Petersen; Rosmarinic acid: new aspects. Phytochemistry Reviews 2013, 12, 207-227, 10.1007/s11101-013-9282-8.
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.-M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2020, 59, 1275–1293.
- Ziauddeen, N.; Rosi, A.; Del Rio, D.; Amoutzopoulos, B.; Nicholson, S.; Page, P.; Scazzina, F.; Brighenti, F.; Ray, S.; Mena, P. Dietary intake of (poly)phenols in children and adults: Cross-sectional analysis of UK National Diet and Nutrition Survey Rolling Programme (2008–2014). Eur. J. Nutr. 2019, 58, 3183–3198.
- Radtke, J.; Linseisen, J.; Wolfram, G. Phenolic acid intake of adults in a Bavarian subgroup of the national food consumption survey. Z. Ernahrungswiss. 1998, 37, 190–197.
- Herrmann, K.P. Occurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Crit. Rev. Food Sci. Nutr. 1989, 28, 315–347.
- Shahidi, F.; Varatharajan, V.; Oh, W.Y.; Peng, H. Phenolic compounds in agri-food by-products, their bioavailability and health effects. J. Food Bioact. 2019, 5, 57–119.
- Ricci, A.; Parpinello, G.P.; Versari, A. The Nutraceutical impact of polyphenolic composition in commonly consumed green tea, green coffee and red wine beverages: A review. Recent Adv. Food Sci. Nutr. Res. 2018, 1, 12–27.
- El-Seedi, H.; Taher, E.; Sheikh, B.; Anjum, S.; Saeed, A.; Alajmi, M.; Moustafa, M.; Al-Mousawi, S.; Farag, M.; Hegazy, M.E.; et al. Chapter 8-hydroxycinnamic acids: Natural sources, biosynthesis, possible biological activities, and roles in islamic medicine. In Studies in Natural Products Chemistry; Atta-Ur-Rahman, Ed.; Elsevier B.V: Amsterdam, The Netherlands, 2018; pp. 269–292.
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.-K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895.
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542.
- Sylvain Guyot; Nathalie Marnet; Djamel Laraba; Philippe Sanoner; Jean-François Drilleau; Reversed-Phase HPLC following Thiolysis for Quantitative Estimation and Characterization of the Four Main Classes of Phenolic Compounds in Different Tissue Zones of a French Cider Apple Variety (Malus domesticaVar. Kermerrien). Journal of Agricultural and Food Chemistry 1998, 46, 1698-1705, 10.1021/jf970832p.
- Hasna El Gharras; Polyphenols: food sources, properties and applications - a review. International Journal of Food Science & Technology 2009, 44, 2512-2518, 10.1111/j.1365-2621.2009.02077.x.
- Subramani Sellappan; Casimir C. Akoh; Gerard Krewer; Phenolic Compounds and Antioxidant Capacity of Georgia-Grown Blueberries and Blackberries. Journal of Agricultural and Food Chemistry 2002, 50, 2432-2438, 10.1021/jf011097r.
- Yuegang Zuo; Chengxia Wang; Jian Zhan; Separation, Characterization, and Quantitation of Benzoic and Phenolic Antioxidants in American Cranberry Fruit by GC−MS. Journal of Agricultural and Food Chemistry 2002, 50, 3789-3794, 10.1021/jf020055f.
- Prashant Kaushik; Isabel Andújar; Santiago Vilanova; Mariola Plazas; Pietro Gramazio; Francisco J. Herraiz; Navjot Singh Brar; Jaime Prohens; Breeding Vegetables with Increased Content in Bioactive Phenolic Acids. Molecules 2015, 20, 18464-18481, 10.3390/molecules201018464.
- María Isabel Alarcón-Flores; Roberto Romero-González; José Luis Martínez Vidal; Antonia Garrido Frenich; Determination of Phenolic Compounds in Artichoke, Garlic and Spinach by Ultra-High-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Food Analytical Methods 2014, 7, 2095-2106, 10.1007/s12161-014-9852-4.
- Adriana Farah; Carmen Marino Donangelo; Phenolic compounds in coffee. Brazilian Journal of Plant Physiology 2006, 18, 23-36, 10.1590/s1677-04202006000100003.
- Adriana Farah; Juliana De Paula Lima; Consumption of Chlorogenic Acids through Coffee and Health Implications. Beverages 2019, 5, 11, 10.3390/beverages5010011.
- Michael N Clifford; Chlorogenic acids and other cinnamates – nature, occurrence and dietary burden. Journal of the Science of Food and Agriculture 1999, 79, 362-372, 10.1002/(sici)1097-0010(19990301)79:3<362::aid-jsfa256>3.3.co;2-4.
- Lauren M. Schopp; Jungmin Lee; J.P. Osborne; Stuart Chescheir; Charles G. Edwards; Metabolism of Nonesterified and Esterified Hydroxycinnamic Acids in Red Wines by Brettanomyces bruxellensis. Journal of Agricultural and Food Chemistry 2013, 61, 11610-11617, 10.1021/jf403440k.
- Yoji Hayasaka; Cory A. Black; Jeremy Hack; Paul Smith; Structural characterization of reaction products of caftaric acid and bisulfite present in a commercial wine using high resolution mass spectrometric and nuclear magnetic resonance techniques. Food Chemistry 2017, 230, 99-107, 10.1016/j.foodchem.2017.03.005.
- Natalia Nowacka-Jechalke; Renata Nowak; Marta Drozd; Marta Olech; Renata Los; Anna Malm; Analysis of phenolic constituents, antiradical and antimicrobial activity of edible mushrooms growing wild in Poland. LWT 2014, 59, 689-694, 10.1016/j.lwt.2014.05.041.
- Maarit J. Rein; Mathieu Renouf; Cristina Cruz‐Hernandez; Lucas Actis‐Goretta; Sagar K. Thakkar; Marcia Da Silva Pinto; Bioavailability of bioactive food compounds: a challenging journey to bioefficacy. British Journal of Clinical Pharmacology 2013, 75, 588-602, 10.1111/j.1365-2125.2012.04425.x.
- J C Espín; Maria T Garcia-Conesa; Francisco A. Tomás-Barberán; Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986-3008, 10.1016/j.phytochem.2007.09.014.
- Tsopmo, A. Chapter 43-processing oats and bioactive components. In Processing and Impact on Active Components in Food; Preedy, V.R., Ed.; Academic Press (Elsevier): Cambridge, MA, USA, 2014; pp. 361–368
- Nuria Mateo Anson; Anna-Marja Aura; Emilia Selinheimo; Ismo Mattila; Kaisa Poutanen; Robin Van Den Berg; Robert Havenaar; Aalt Bast; Guido R.M.M. Haenen; Bioprocessing of Wheat Bran in Whole Wheat Bread Increases the Bioavailability of Phenolic Acids in Men and Exerts Antiinflammatory Effects ex Vivo. The Journal of Nutrition 2010, 141, 137-143, 10.3945/jn.110.127720.
- Norma Julieta Salazar-López; Gustavo A. González-Aguilar; Ofelia Rouzaud-Sández; Maribel Robles-Sánchez; Bioaccessibility of hydroxycinnamic acids and antioxidant capacity from sorghum bran thermally processed during simulated in vitro gastrointestinal digestion. Journal of Food Science and Technology 2018, 55, 2021-2030, 10.1007/s13197-018-3116-z.
- Stéphanie Galland; Njara Rakotomanomana; Claire Dufour; Nathalie Mora; Olivier Dangles; Synthesis of hydroxycinnamic acid glucuronides and investigation of their affinity for human serum albumin. Organic & Biomolecular Chemistry 2008, 6, 4253, 10.1039/b809965k.
- Stalmach, A. Chapter 42-Bioavailability of dietary anthocyanins and hydroxycinnamic acids. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 561–576
- Sophie Lafay; Christine Morand; Claudine Manach; Catherine Besson; Augustin Scalbert; Absorption and metabolism of caffeic acid and chlorogenic acid in the small intestine of rats.. British Journal of Nutrition 2006, 96, 39-46, 10.1079/bjn20061714.
- Zhaohui Zhao; Mohammed H. Moghadasian; Bioavailability of hydroxycinnamates: a brief review of in vivo and in vitro studies. Phytochemistry Reviews 2009, 9, 133-145, 10.1007/s11101-009-9145-5.
- Couteau, D.; McCartney, A.L.; Gibson, G.R.; Williamson, G.; Faulds, C.B. Isolation and characterization of human colonic bacteria able to hydrolyse chlorogenic acid. J. Appl. Microbiol. 2001, 90, 873–881.
- Poquet, L.; Clifford, M.N.; Williamson, G. Transport and metabolism of ferulic acid through the colonic epithelium. Drug Metab. Dispos. Biol. Fate Chem. 2008, 36, 190–197
- Yutaka Konishi; Makoto Shimizu; Transepithelial Transport of Ferulic Acid by Monocarboxylic Acid Transporter in Caco-2 Cell Monolayers. Bioscience, Biotechnology, and Biochemistry 2003, 67, 856-862, 10.1271/bbb.67.856.
- Yutaka Konishi; Shoko Kobayashi1); Makoto Shimizu; Transepithelial Transport ofp-Coumaric Acid and Gallic Acid in Caco-2 Cell Monolayers. Bioscience, Biotechnology, and Biochemistry 2003, 67, 2317-2324, 10.1271/bbb.67.2317.
- Yutaka Konishi; Shoko Kobayashi; Transepithelial Transport of Chlorogenic Acid, Caffeic Acid, and Their Colonic Metabolites in Intestinal Caco-2 Cell Monolayers. Journal of Agricultural and Food Chemistry 2004, 52, 2518-2526, 10.1021/jf035407c.
- Yutaka Konishi; Yoshitaka Hitomi; Michiko Yoshida; Eiji Yoshioka; Pharmacokinetic Study of Caffeic and Rosmarinic Acids in Rats after Oral Administration. Journal of Agricultural and Food Chemistry 2005, 53, 4740-4746, 10.1021/jf0478307.
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71.
- Omar, M.H.; Mullen, W.; Stalmach, A.; Auger, C.; Rouanet, J.-M.; Teissedre, P.-L.; Caldwell, S.T.; Hartley, R.C.; Crozier, A. Absorption, disposition, metabolism, and excretion of [3-14C]caffeic acid in rats. J. Agric. Food Chem. 2012, 60, 5205–5214.
- Wang, S.-J.; Zeng, J.; Yang, B.-K.; Zhong, Y.-M. Bioavailability of caffeic acid in rats and its absorption properties in the Caco-2 cell model. Pharm. Biol. 2014, 52, 1150–1157.
- Geoff W Plumb; Maria T Garcia‐Conesa; Paul A. Kroon; Mike Rhodes; Saxon Ridley; Gary Williamson; Metabolism of chlorogenic acid by human plasma, liver, intestine and gut microflora†. Journal of the Science of Food and Agriculture 1999, 79, 390-392, 10.1002/(sici)1097-0010(19990301)79:3<390::aid-jsfa258>3.3.co;2-s.
- Kehan Pei; Juanying Ou; Junqing Huang; Shiyi Ou; p -Coumaric acid and its conjugates: dietary sources, pharmacokinetic properties and biological activities. Journal of the Science of Food and Agriculture 2016, 96, 2952-2962, 10.1002/jsfa.7578.
- Muhammad Islam; Naureen Shehzadi; Muhammad Salman; Fakhra Zahid; Humaira M. Khan; Sohail Amjad; Muhammad Z. Danish; Nadeem I. Bukhari; Khalid Hussain; Pharmacokinetics of Caffeic Acid from Methanol Seed Extract of Syzygium cumini L in Rats. Tropical Journal of Pharmaceutical Research 2016, 15, 363, 10.4314/tjpr.v15i2.20.
- Yumiko Yamada; Hiroyuki Yasui; Hiromu Sakurai; Suppressive Effect of Caffeic Acid and its Derivatives on the Generation of UVA?induced Reactive Oxygen Species in the Skin of Hairless Mice and Pharmacokinetic Analysis on Organ Distribution of Caffeic Acid in ddY Mice. Photochemistry and Photobiology 2006, 82, 1668-1676, 10.1111/j.1751-1097.2006.tb09829.x.
- Chenning Zhang; Weidong Ma; Yonghong Zhang; Qibin Wang; Caibin Qin; Shiming Du; Liangyong Huang; Fang Ye; Li Chen; Tao Zheng; et al. Pharmacokinetics, Bioavailability, and Tissue Distribution Study of Angoroside C and Its Metabolite Ferulic Acid in Rat Using UPLC-MS/MS. Frontiers in Pharmacology 2018, 9, 1186, 10.3389/fphar.2018.01186.
- Mattia Gasperotti; Sabina Passamonti; Federica Tramer; Domenico Masuero; Graziano Guella; Fulvio Mattivi; Urska Vrhovsek; Fate of Microbial Metabolites of Dietary Polyphenols in Rats: Is the Brain Their Target Destination?. ACS Chemical Neuroscience 2015, 6, 1341-1352, 10.1021/acschemneuro.5b00051.
- Yulu Zhou; Ting Zhou; Qi Pei; Shikun Liu; Hong Yuan; Pharmacokinetics and Tissue Distribution Study of Chlorogenic Acid from Lonicerae Japonicae Flos Following Oral Administrations in Rats. Evidence-Based Complementary and Alternative Medicine 2014, 2014, 1-7, 10.1155/2014/979414.
- Gaurav Kumar; Pankaj Paliwal; Sumedha Mukherjee; Nishant Patnaik; Sairam Krishnamurthy; Ranjana Patnaik; Pharmacokinetics and brain penetration study of chlorogenic acid in rats. Xenobiotica 2018, 49, 339-345, 10.1080/00498254.2018.1445882.
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharm. 2017, 139, 82–93.
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513.
- Nandita Shangari; Tom S. Chan; Peter J. O'brien; Sulfation and Glucuronidation of Phenols: Implications in Coenyzme Q Metabolism. Methods in Enzymology 2005, 400, 342-359, 10.1016/s0076-6879(05)00020-0.
- Chi Chun Wong; Walter Meinl; Hans-Rudolf Glatt; Denis Barron; Angelique Stalmach; Heike Steiling; Alan Crozier; Gary Williamson; In vitro and in vivo conjugation of dietary hydroxycinnamic acids by UDP-glucuronosyltransferases and sulfotransferases in humans. The Journal of Nutritional Biochemistry 2010, 21, 1060-1068, 10.1016/j.jnutbio.2009.09.001.
- M.F. Andreasen; Paul A. Kroon; Gary Williamson; Maria T Garcia-Conesa; Esterase activity able to hydrolyze dietary antioxidant hydroxycinnamates is distributed along the intestine of mammals.. Journal of Agricultural and Food Chemistry 2001, 49, 5679-5684, 10.1021/jf010668c.
- Michael N. Clifford; Indu B. Jaganath; Iziar Amaia Ludwig; Alan Crozier; Chlorogenic acids and the acyl-quinic acids: discovery, biosynthesis, bioavailability and bioactivity. Natural Product Reports 2017, 34, 1391-1421, 10.1039/c7np00030h.
- Michael N. Clifford; Asimina Kerimi; Gary Williamson; Bioavailability and metabolism of chlorogenic acids (acyl-quinic acids) in humans. Comprehensive Reviews in Food Science and Food Safety 2020, 19, 1299–1352, 10.1111/1541-4337.12518.
- Sandra M. Kern; Richard N. Bennett; Paul W. Needs; Fred A. Mellon; Paul Kroon; Maria T Garcia-Conesa; Characterization of Metabolites of Hydroxycinnamates in the in Vitro Model of Human Small Intestinal Epithelium Caco-2 Cells. Journal of Agricultural and Food Chemistry 2003, 51, 7884-7891, 10.1021/jf030470n.
- Stalmach, A.; Steiling, H.; Williamson, G.; Crozier, A. Bioavailability of chlorogenic acids following acute ingestion of coffee by humans with an ileostomy. Arch. Biochem. Biophys. 2010, 501, 98–105.
- Stalmach, A.; Mullen, W.; Barron, D.; Uchida, K.; Yokota, T.; Cavin, C.; Steiling, H.; Williamson, G.; Crozier, A. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: Identification of biomarkers of coffee consumption. Drug Metab. Dispos. 2009, 37, 1749–1758.
- Raquel Mateos; Luis Goya; Laura Bravo; Uptake and Metabolism of Hydroxycinnamic Acids (Chlorogenic, Caffeic, and Ferulic Acids) by HepG2 Cells as a Model of the Human Liver. Journal of Agricultural and Food Chemistry 2006, 54, 8724-8732, 10.1021/jf061664g.
- Zhaohui Zhao; Yukari Egashira; Hiroo Sanada; Ferulic acid sugar esters are recovered in rat plasma and urine mainly as the sulfoglucuronide of ferulic acid.. The Journal of Nutrition 2003, 133, 1355-1361, 10.1093/jn/133.5.1355.
- Miren Gómez-Juaristi; Sara Martínez-López; Beatriz Sarria; Laura Bravo; Raquel Mateos; Absorption and metabolism of yerba mate phenolic compounds in humans. Food Chemistry 2018, 240, 1028-1038, 10.1016/j.foodchem.2017.08.003.
- Marie-Paule Gonthier; Marie-Anne Verny; Catherine Besson; Christian Rémésy; Augustin Scalbert; Chlorogenic Acid Bioavailability Largely Depends on Its Metabolism by the Gut Microflora in Rats. The Journal of Nutrition 2003, 133, 1853-1859, 10.1093/jn/133.6.1853.
- A N Booth; O H Emerson; F T Jones; F Deeds; Urinary metabolites of caffeic and chlorogenic acids.. Journal of Biological Chemistry 1957, 229, 51–59.
- Sadeghi Ekbatan, S.; Iskandar, M.M.; Sleno, L.; Sabally, K.; Khairallah, J.; Prakash, S.; Kubow, S. Absorption and metabolism of phenolics from digests of polyphenol-rich potato extracts using the Caco-2/HepG2 co-culture system. Foods 2018, 7, 8.
- Sadeghi Ekbatan, S.; Sleno, L.; Sabally, K.; Khairallah, J.; Azadi, B.; Rodes, L.; Prakash, S.; Donnelly, D.J.; Kubow, S. Biotransformation of polyphenols in a dynamic multistage gastrointestinal model. Food Chem. 2016, 204, 453–462.
- Massimo D'archivio; Carmela Filesi; Roberta Di Benedetto; Raffaella Gargiulo; C. Giovannini; R. Masella; Polyphenols, dietary sources and bioavailability.. Annali dellIstituto Superiore di Sanità 2007, 43, 348–361.
- Farah, A.; Duarte, G. Chapter 87-Bioavailability and metabolism of chlorogenic acids from coffee. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 789–801.
- Stalmach, A.; Williamson, G.; Crozier, A. Impact of dose on the bioavailability of coffee chlorogenic acids in humans. Food Funct. 2014, 5, 1727–1737.
- Stalmach, A.; Williamson, G.; Clifford, M.N. Dietary hydroxycinnamates and their bioavailability. In Flavonoids and Related Compounds: Bioavailability and Function; Spencer, J.P.E., Crozier, A., Eds.; CRC Press, Taylor and Francis Group: New York, NY, USA, 2012; pp. 123–156.
- Choudhury, R.; Srai, S.K.; Debnam, E.; Rice-Evans, C.A. Urinary excretion of hydroxycinnamates and flavonoids after oral and intravenous administration. Free Radic. Biol. Med. 1999, 27, 278–286.
- Bourne, L.C.; Rice-Evans, C. Bioavailability of ferulic acid. Biochem. Biophys. Res. Commun. 1998, 253, 222–227
- Kunihiro Kishida; Harumi Matsumoto; Urinary excretion rate and bioavailability of chlorogenic acid, caffeic acid, p-coumaric acid, and ferulic acid in non-fasted rats maintained under physiological conditions.. Heliyon 2019, 5, e02708, 10.1016/j.heliyon.2019.e02708.
- Manuel Y. Schär; Giulia Corona; Gulten Soycan; Clemence Dine; Angelika Kristek; Sarah N. S. Alsharif; Volker Behrends; Alison Lovegrove; Peter R. Shewry; Jeremy P.E. Spencer; et al. Excretion of Avenanthramides, Phenolic Acids and their Major Metabolites Following Intake of Oat Bran. Molecular Nutrition & Food Research 2017, 62, 1700499, 10.1002/mnfr.201700499.
- Farah, A.; de Paula Lima, J. Chapter 16: Chlorogenic acids: Daily consumption through coffee, metabolism and potential health effects. In Coffee: Consumption and Health Implications; Farah, A., Ed.; Royal Society of Chemistry Publishing: Cambridge, UK, 2019; pp. 364–415.
- Adomako-Bonsu, A.G.; Chan, S.L.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicology 2017, 40, 248–255.
- Martínez-Huélamo, M.; Vallverdú-Queralt, A.; Lecce, G.D.; Valderas-Martínez, P.; Tulipani, S.; Jáuregui, O.; Escribano-Ferrer, E.; Estruch, R.; Illan, M.; Lamuela-Raventós, R.M. Bioavailability of tomato polyphenols is enhanced by processing and fat addition: Evidence from a randomized feeding trial. Mol. Nutr. Food Res. 2016, 60, 1578–1589.
