Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Amin Gasmi and Version 2 by Conner Chen.
Dental implants to replace lost teeth are a common dentistry practice nowadays. Titanium dental implants display a high success rate and improved safety profile. Nevertheless, there is an increasing peri-implantitis (PI), an inflammatory disease associated with polymicrobial infection that adversely affects the hard and soft tissues around the implant. Studies have demonstrated that oral microbiota (microorganisms residing in the oral cavity collectively known as oral microbiota) associated with periodontitis (PE) is involved in the infections related to PI, indicating a common link between PE and PI.
- dental implants
- inflammation
- peri-implantitis
1. Introduction
Dental issues are common health concerns that require immediate attention to relieve excruciating pain and prevent tooth deterioration [1]. There is a close association between oral and dental health, in which maintaining good oral hygiene directly links with better dental health [2]. According to an estimation, around 267 million people around the world are suffering from tooth loss [3]. The permanent tooth loss and alveolar bone defects can be managed by moveable dentures that are fixed in soft tissues and need to be changed over time. Now, dental implants have emerged as the preferred procedure for permanent tooth loss treatment and replaced the use of dentures. Biocompatibility and low cost of titanium dental implants make it the most often-used choice. Titanium is a bioinert substance that has little to no negative effects on the tissue it is adhered [4][5][6][4,5,6]. Despite all their usefulness, dental implants can sometimes cause infection. Improper implant placement or osseointegration failure can trigger the host inflammatory response and lead to the development of peri-implantitis (PI), which has become a growing concern in dentistry because of the lack of effective treatment strategies [7].
Generally, PI was defined as an inflammatory process affecting both soft and hard tissues surrounding an osseointegrated implant, associated with suppuration or bleeding after gentle probing, resulting in quick loss of supporting bone [8]. PI can limit dental implants’ clinical success and impose health and financial burdens on patients [9][10][9,10]. There are multiple causes of PI depending on the type of implant used and the overall health status of the patient such as poor oral hygiene, smoking, diabetes, history of periodontal disease, and previous implant loss [11][12][11,12]. According to Papi et al. [13], a higher risk of PI was observed in people with hyperglycemia.
Metabolic syndrome is a wide spectrum of health disorders, including hyperglycemia, dyslipidemia, visceral obesity, rheumatoid arthritis hypertension, etc. [14]. There is no doubt that the presence of metabolic disorders while studying implant engraftment in animals and patients complicates the process of survival of implants and increases the risk of PI relative to the healthy population [14][15][16][14,15,16]. In this regard, the frequency of PI increases strongly in older patients [17]. High life expectancy and reliability of modern implant dentistry are all factors that lead to the increased percentage of dental implants in elderly patients and risk an increase in the number of side effects, i.e., PI [13].
The inflammatory reactions in PI affect the surrounding tissues of the implant, where the high levels of pro-inflammatory cytokines such as interleukin 1beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) promote bone resorption with subsequent adverse health consequences [18]. The colonized bacteria and their biofilms formation on the implants are the significant causes of peri-implant tissue inflammation due to their interactions with the host immune system. Streptococcus sanguinis, Streptococcus mitis, Streptococcus oralis and actinomyces are some common bacterial species that colonize at an early stage of biofilm formation while Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia are some late colonizers [19][20][21][19,20,21]. With the recent increasing practice of dental implants, there are parallel increases in dental implant rejection and PI cases in dentistry practices. PI therapies using biomaterials such as fibers, gels, beads, and regeneration membranes to deliver antibiotics have been effectively applied in recent years.
The crucial mediators for PI are local tissue inflammation and increased oxidative stress [22]. These mediators are also the common determinations in most systemic metabolic conditions, such as type 2 diabetes mellitus and dyslipidemia [23]. As it is known, hormones coordinate different physiological processes in the body, including various metabolic conditions, growth, and development [24]. There is no doubt that bone physiology and the repair process for implant osseointegration strongly depend on the body’s hormonal status. Martin et al. [25] conducted the transcriptome-wide gene expression analysis and found the mechanisms of upregulation of genes in the endosomal-lysosomal and oxidative stress pathway in PI. They suggested that a crucial role in PI could be the receptor-driven responses to extracellular signals, such as implant-derived titanium particles. Through the transcriptome analysis, Cho et al. [23] found that smoking differentially affected PI and periodontitis (PE) in terms of host-defense mechanism impairment.
Several systemic metabolic diseases reduce the long-term success rate of dental implants. A 16-year follow-up study reported the success rate at only 82.9%, with around 5–8% of the failed osseointegration from PI [5]. Several reports indicate associations between chronic local dental inflammation, such as PE, and the increased risks of many systemic conditions, including metabolic diseases, cardiovascular diseases, cerebrovascular diseases, and neurodegenerative disorders [23][26][27][28][29][23,26,27,28,29]. The incidence of peri-implantitis patients with chronic PE is 4–5 times greater than those without a burdened periodontal history [9][30][9,30].
There are bi-directional relationships between local dental disease and systemic metabolic disorders, as shown in Figure 1. These relationships could be the crucial determinant for the success of the dental implant procedure and PI treatment. While planning and treating the local pathological oral conditions are necessary, the consideration and proper management of underlying metabolic disorders could determine the practice outcomes [5][22][31][32][33][34][5,22,31,32,33,34].
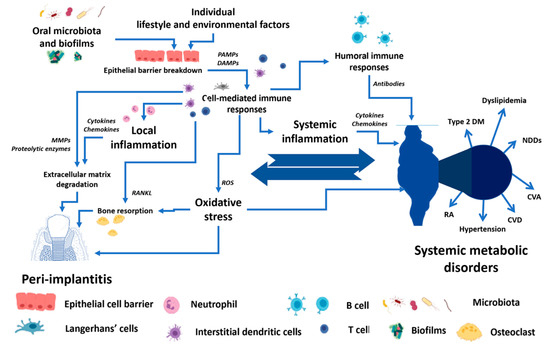
Figure 1. The conceptual framework of the bi-directional relationship between peri-implantitis and systemic metabolic disorders. PAMPs—Pathogen-associated molecular patterns, DAMPs—Damage-associated molecular patterns, MMPs—Matrix metalloproteinases, RANKL—Receptor activator of nuclear factor-κB ligand, ROS—Reactive oxygen species, Type 2 DM—Type 2 diabetes mellitus, NDDS—Neurodegenerative disorders, CVA—Cerebrovascular accident, CVD—Cardiovascular diseases, RA—Rheumatoid arthritis.
