Increasingly popular, ultra-endurance participation exposes athletes to extremely high levels of functional and structural damage. Ultra-endurance athletes commonly develop acute kidney injury (AKI) and other pathologies harmful to kidney health. There is strong evidence that non-steroidal anti-inflammatory drugs, common amongst ultra-athletes, is linked to increased risk and severity of AKI and potentially ischaemic renal injury, i.e., acute tubular necrosis. Ultra-endurance participation also increases the risk of exertional rhabdomyolysis, exercise-associated hyponatremia, and gastrointestinal symptoms, interlinked pathologies all with potential to increase the risk of AKI. Hydration and fuelling both also play a role with the development of multiple pathologies and ultimately AKI, highlighting the need for individualised nutritional and hydration plans to promote athlete health. Faster athletes, supplementing nitrates, and being female also increase the risk of developing AKI in this setting.
- ultra-endurance
- acute kidney injury
- non-steroidal anti-inflammatory drugs
- hydration
- exertional rhabdomyolysis
- exercise-associated hyponatremia
1. Introduction
2. Acute Kidney Injury and Ultra-Endurance Events
 = increase,
= increase,  = decrease.
= decrease.3. Non-Steroidal Anti-Inflammatory Drugs
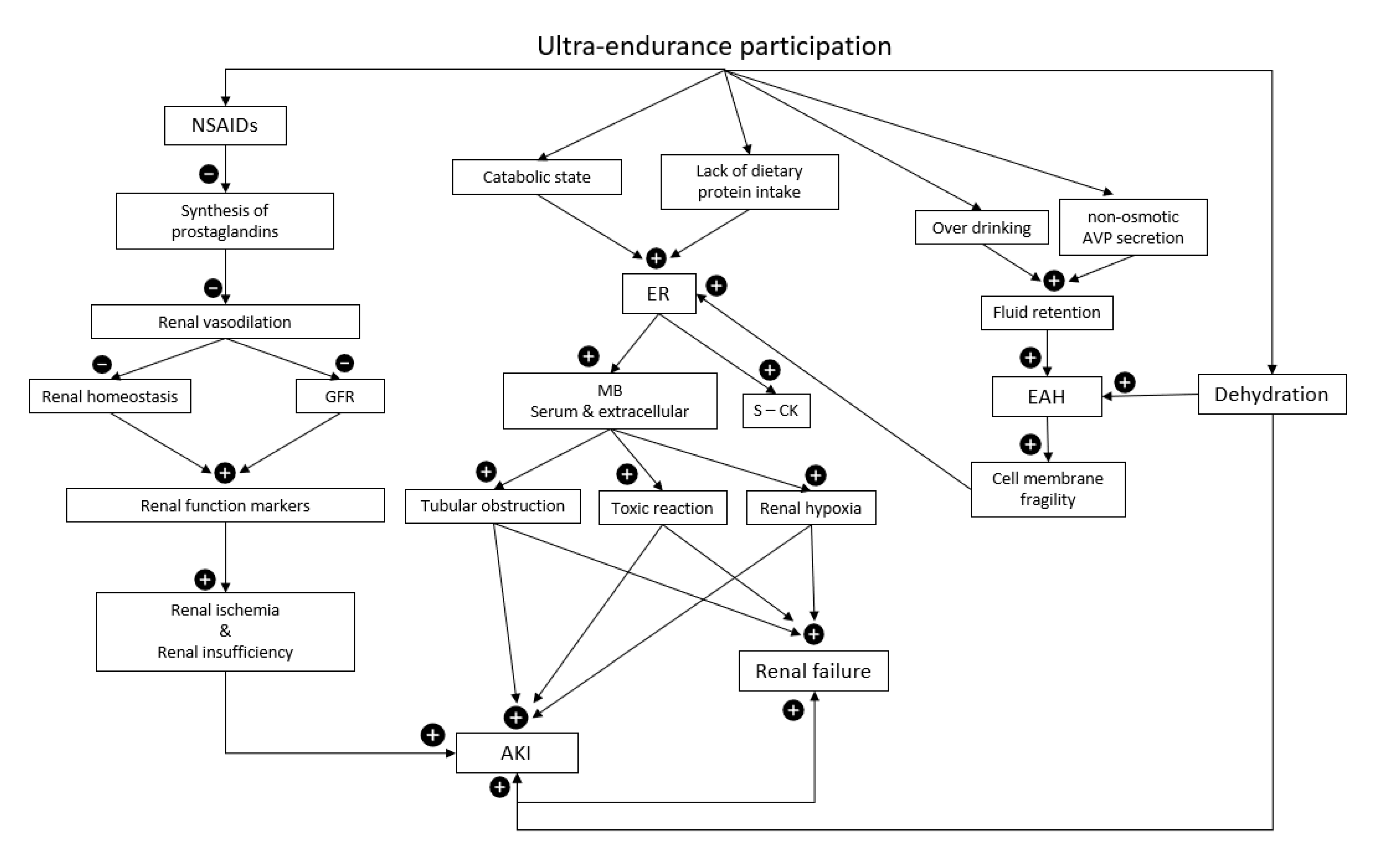
 = increase,
= increase,  = decrease.
= decrease.4. Hydration
5. Exertional Rhabdomyolysis
References
- Scheer, V.; Tiller, N.B.; Doutreleau, S.; Khodaee, M.; Knechtle, B.; Pasternak, A.; Rojas-Valverde, D. Potential Long-Term Health Problems Associated with Ultra-Endurance Running: A Narrative Review. Sport Med. 2021, 52, 725–740.
- Rubio-arias, J.Á.; Ávila-Gandía, V.; López-román, F.J.; Soto-méndez, F.; Alcaraz, P.E.; Ramos-campo, D.J. Muscle damage and inflammation biomarkers after two ultra-endurance mountain races of different distances: 54 km vs 111 km. Physiol. Behav. 2019, 205, 51–57.
- Lecina, M.; López, I.; Castellar, C.; Pradas, F. Extreme ultra-trail race induces muscular damage, risk for acute kidney injury and hyponatremia: A case report. Int. J. Environ. Res. Public Health 2021, 18, 11323.
- Rojas-Valverde, D.; Sánchez-Ureña, B.; Crowe, J.; Timón, R.; Olcina, G.J. Exertional rhabdomyolysis and acute kidney injury in endurance sports: A systematic review. Eur. J. Sport Sci. 2021, 21, 261–274.
- Riebe, D.; Franklin, B.A.; Thompson, P.D.; Garber, C.E.; Whitfield, G.P.; Magal, M.; Pescatello, L.S. Updating ACSM’s recommendations for exercise preparticipation health screening. Med. Sci. Sport. Exerc. 2015, 47, 2473–2479.
- Mansour, S.; Verma, G.; Pata, R.; Martin, T.; Perazella, M.; Parikh, C. Kidney Injury and Repair Biomarkers in Marathon Runners. Am. J. Kidney Dis. 2017, 70, 252–261.
- Scheer, V.; Rojas-Valverde, D. Long-term health issues in ultraendurance runners: Should we be concerned? BMJ Open Sport Exerc. Med. 2021, 7, e001131.
- Hodgson, L.E.; Walter, E.; Venn, R.M.; Galloway, R.; Pitsiladis, Y.; Sardat, F.; Forni, L.G. Acute kidney injury associated with endurance events—Is it a cause for concern? A systematic review. BMJ Open Sport Exerc. Med. 2017, 3, e000093.
- Rojas-Valverde, D.; Olcina, G.; Sánchez-Ureña, B.; Pino-Ortega, J.; Martínez-Guardado, I.; Timón, R. Proteinuria and bilirubinuria as potential risk indicators of acute kidney injury during running in outpatient settings. Medicina 2020, 56, 562.
- Hewing, B.; Schattke, S.; Spethmann, S.; Sanad, W.; Schroeckh, S.; Schimke, I.; Halleck, F.; Peters, H.; Brechtel, L.; Lock, J.; et al. Cardiac and renal function in a large cohort of amateur marathon runners. Cardiovasc. Ultrasound 2015, 13, 13.
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147.
- Cardinale, D.A.; Lilja, M.; Mandić, M.; Gustafsson, T.; Larsen, F.J.; Lundberg, T.R. Resistance training with co-ingestion of anti-inflammatory drugs attenuates mitochondrial function. Front. Physiol. 2017, 8, 1074.
- Omeragic, E.; Marjanovic, A.; Djedjibegovic, J.; Turalic, A.; Dedic, M.; Niksic, H.; Lugusic, A.; Sober, M. Prevalence of use of permitted pharmacological substances for recovery among athletes. Pharmacia 2021, 68, 35–42.
- Lipman, G.S.; Shea, K.; Christensen, M.; Phillips, C.; Burns, P.; Higbee, R.; Koskenoja, V.; Eifling, K.; Krabak, B.J. Ibuprofen versus placebo effect on acute kidney injury in ultramarathons: A randomised controlled trial. Emerg. Med. J. 2017, 34, 637–642.
- Nieman, D.C.; Henson, D.A.; Dumke, C.L.; Oley, K.; McAnulty, S.R.; Davis, J.M.; Murphy, E.A.; Utter, A.C.; Lind, R.H.; McAnulty, L.S.; et al. Ibuprofen use, endotoxemia, inflammation, and plasma cytokines during ultramarathon competition. Brain Behav. Immun. 2006, 20, 578–584.
- Hoffman, M.D.; Fogard, K. Factors related to successful completion of a 161-km ultramarathon. Int. J. Sports Physiol. Perform. 2011, 6, 25–37.
- Martínez, S.; Aguiló, A.; Moreno, C.; Lozano, L.; Tauler, P. Use of non-steroidal anti-inflammatory drugs among participants in a mountain ultramarathon event. Sports 2017, 5, 11.
- Gorski, T.; Cadore, E.L.; Pinto, S.S.; da Silva, E.M.; Correa, C.S.; Beltrami, F.G.; Kruel, L.M. Use of NSAIDs in triathletes: Prevalence, level of awareness and reasons for use. Br. J. Sports Med. 2011, 45, 85–90.
- Steckling, F.M.; Lima, F.D.; Farinha, J.B.; Rosa, P.C.; Royes, L.F.F.; Cuevas, M.J.; Bresciani, G.; Soares, F.A.; González-Gallego, J.; Barcelos, R.P. Diclofenac attenuates inflammation through TLR4 pathway and improves exercise performance after exhaustive swimming. Scand. J. Med. Sci. Sports 2020, 30, 264–271.
- Lundberg, T.R.; Howatson, G. Analgesic and anti-inflammatory drugs in sports: Implications for exercise performance and training adaptations. Scand. J. Med. Sci. Sports 2018, 28, 2252–2262.
- Lilja, M.; Mandić, M.; Apró, W.; Melin, M.; Olsson, K.; Rosenborg, S.; Gustafsson, T.; Lundberg, T.R. High doses of anti-inflammatory drugs compromise muscle strength and hypertrophic adaptations to resistance training in young adults. Acta Physiol. 2018, 222, e12948.
- Nderitu, P.; Doos, L.; Jones, P.W.; Davies, S.J.; Kadam, U.T. Non-steroidal anti-inflammatory drugs and chronic kidney disease progression: A systematic review. Fam. Pract. 2013, 30, 247–255.
- Hou, S.K.; Chiu, Y.H.; Tsai, Y.F.; Tai, L.C.; Hou, P.C.; How, C.K.; Yang, C.C.; Kao, W.F. Clinical impact of speed variability to identify ultramarathon runners at risk for acute kidney injury. PLoS ONE 2015, 10, e0133146.
- Bongers, C.C.; Alsady, M.; Nijenhuis, T.; Tulp, A.D.; Eijsvogels, T.M.; Deen, P.M.; Hopman, M.T. Impact of acute versus prolonged exercise and dehydration on kidney function and injury. Physiol. Rep. 2018, 6, e13734.
- Lipman, G.S.; Krabak, B.J.; Rundell, S.D.; Shea, K.M.; Badowski, N.; Little, C. Incidence and prevalence of acute kidney injury during multistage ultramarathons. Clin. J. Sport Med. 2016, 26, 314–319.
- Kao, W.F.; Hou, S.K.; Chiu, Y.H.; Chou, S.L.; Kuo, F.C.; Wang, S.H.; Chen, J.J. Effects of 100-km ultramarathon on acute kidney injury. Clin. J. Sport Med. 2015, 25, 49–54.
- Costa, R.J.S.; Hoffman, M.D.; Stellingwerff, T. Research in Sports Medicine Considerations for ultra-endurance activities: Part 1- nutrition. Res. Sport Med. 2019, 27, 166–181.
- Vitale, K.; Getzin, A. Nutrition and supplement update for the endurance athlete: Review and recommendations. Nutrients 2019, 11, 1289.
- Nikolaidis, P.T.; Veniamakis, E.; Rosemann, T.; Knechtle, B. Nutrition in ultra-endurance: State of the art. Nutrients 2018, 10, 1995.
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568.
- Jeukendrup, A.E.; Jentjens, R.L.P.G.; Moseley, L. Nutritional considerations in triathlon. Sport Med. 2005, 35, 163–181.
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International society of sports nutrition position stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20.
- McCullough, P.A.; Chinnaiyan, K.M.; Gallagher, M.J.; Colar, J.M.; Geddes, T.; Gold, J.M.; Trivax, J.E. Changes in renal markers and acute kidney injury after marathon running. Nephrology 2011, 16, 194–199.
- Bruso, J.R.; Hoffman, M.D.; Rogers, I.R.; Lee, L.; Towle, G.; Hew-butler, T. Rhabdomyolysis and hyponatremia: A cluster of five cases at the 161-km 2009 Western States Endurance Run. Wilderness Environ. Med. 2010, 21, 303–308.
- Boulter, J.; Noakes, T.D.; Hew-Butler, T. Acute renal failure in four Comrades Marathon runners ingesting the same electrolyte supplement: Coincidence or causation? S. Afr. Med. J. 2011, 101, 876–878.
- Stasi, A.; Intini, A.; Divella, C.; Franzin, R.; Montemurno, E.; Grandaliano, G.; Ronco, C.; Fiaccadori, E.; Pertosa, G.B.; Gesualdo, L.; et al. Emerging role of Lipopolysaccharide binding protein in sepsis-induced acute kidney injury. Nephrol. Dial. Transplant. 2017, 32, 24–31.
- Lim, C.L.; Suzuki, K. Systemic inflammation mediates the effects of endotoxemia in the mechanisms of heat stroke. Biol. Med. 2016, 9, 1.
- Makris, K.; Spanou, L. Acute kidney injury: Definition, pathophysiology and clinical phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98.
- Lipman, G.S.; Krabak, B.J.; Waite, B.L.; Logan, S.B.; Menon, A.; Chan, G.K. A prospective cohort study of acute kidney injury in multi-day ultramarathon runners. Wilderness Environ. Med. 2014, 22, 358.
- Shin, K.; Park, K.D.; Ahn, J.; Park, Y.; Kim, Y.-J. Comparison of changes in biochemical markers for skeletal muscles, hepatic metabolism, and renal function after three types of long-distance running: Observational study. Medicine 2016, 95, e3657.
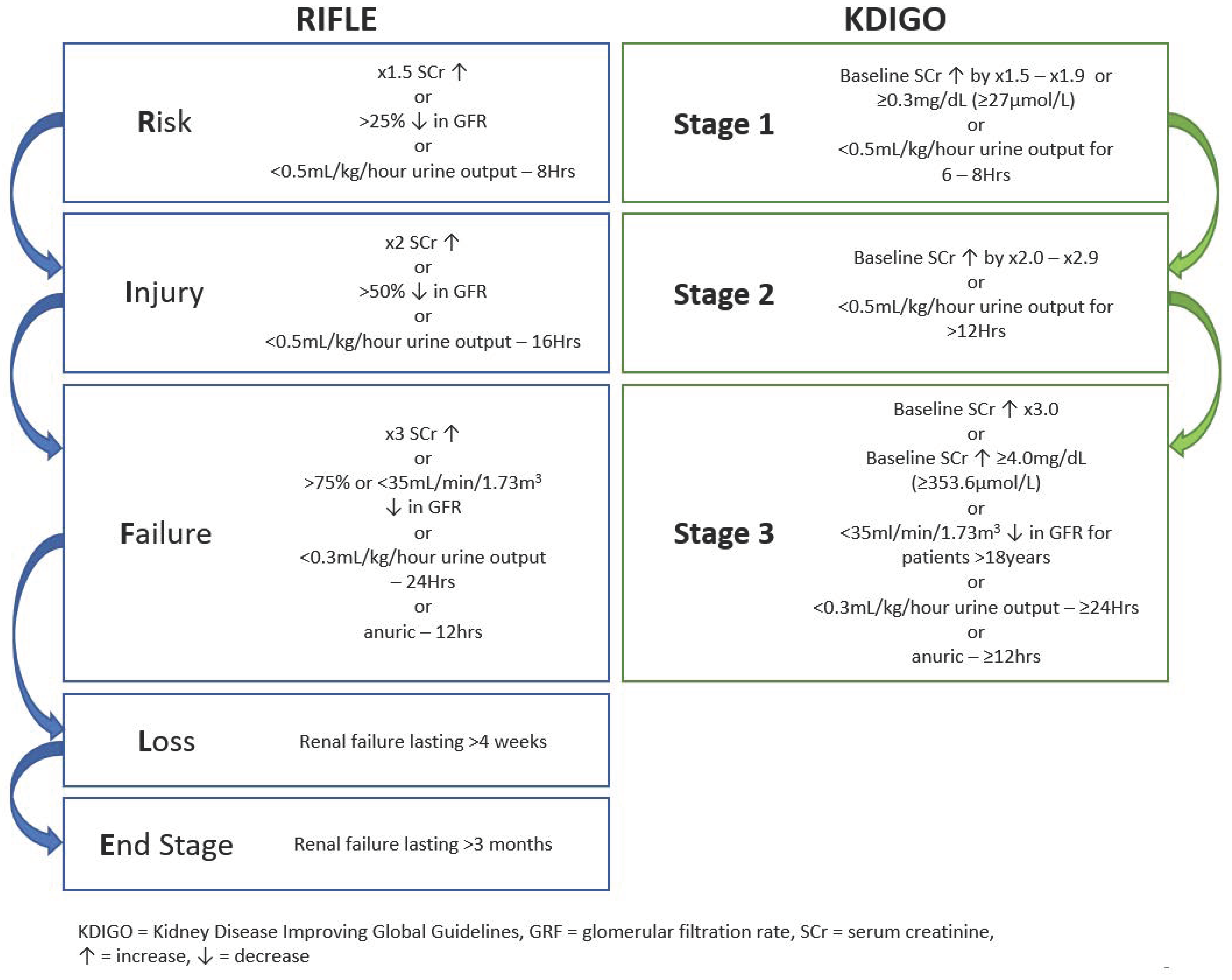
- Lopes, J.A.; Jorge, S. The RIFLE and AKIN classifications for acute kidney injury: A critical and comprehensive review. Clin. Kidney J. 2013, 6, 8–14.
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron. Clin. Pract. 2012, 120, 179–184.
- Khodaee, M.; Irion, B.; Spittler, J.; Saeedi, A.; Hoffman, M.D. Characteristics of runners meeting acute kidney injury criteria following a 161-km ultramarathon. Transl. Sport Med. 2021, 4, 733–740.
- Rojas-Valverde, D.; Martínez-Guardado, I.; Sánchez-Ureña, B.; Timón, R.; Scheer, V.; Pino-Ortega, J.; Olcina, G. Outpatient assessment of mechanical load, heat strain and dehydration as causes of transitional acute kidney injury in endurance trail runners. Int. J. Environ. Res. Public Health 2021, 18, 10217.
- Jouffroy, R.; Lebreton, X.; Mansencal, N.; Anglicheau, D. Acute kidney injury during an ultra-distance race. PLoS ONE 2019, 14, e0222544.
- Poussel, M.; Touzé, C.; Allado, E.; Frimat, L.; Hily, O.; Thilly, N.; Rousseau, H.; Vauthier, J.C.; Chenuel, B. Ultramarathon and renal function: Does exercise-induced acute kidney injury really exist in common conditions? Front. Sport Act Living 2020, 1, 71.
- Chlíbková, D.; Knechtle, B.; Rosemann, T.; Tomášková, I.; Novotný, J.; Žákovská, A.; Uher, T. Rhabdomyolysis and exercise-associated hyponatremia in ultra-bikers and ultra-runners. J. Int. Soc. Sports Nutr. 2015, 12, 29.
- Neumayr, G.; Pfister, R.; Hoertnagl, H.; Mitterbauer, G.; Getzner, W.; Ulmer, H.; Gaenzer, H.; Joannidis, M. The effect of marathon cycling on renal function. Int. J. Sports Med. 2003, 24, 131–137.
- Neumayr, G.; Pfister, R.; Hoertnagl, H.; Mitterbauer, G.; Prokop, W.; Joannidis, M. Renal function and plasma volume following ultramarathon cycling. Int. J. Sports Med. 2005, 26, 2–8.
- Hoffman, M.D.; Stuempfle, K.J.; Fogard, K.; Hew-butler, T.; Winger, J.; Weiss, R.H. Urine dipstick analysis for identification of runners susceptible to acute kidney injury following an ultramarathon. J. Sports Sci. 2013, 31, 20–31.
- Andres, S.; Ziegenhagen, R.; Trefflich, I.; Pevny, S.; Schultrich, K.; Braun, H.; Schänzer, W.; Hirsch-Ernst, K.I.; Schäfer, B.; Lampen, A. Creatine and creatine forms intended for sports nutrition. Mol. Nutr. Food Res. 2017, 61, 1–18.
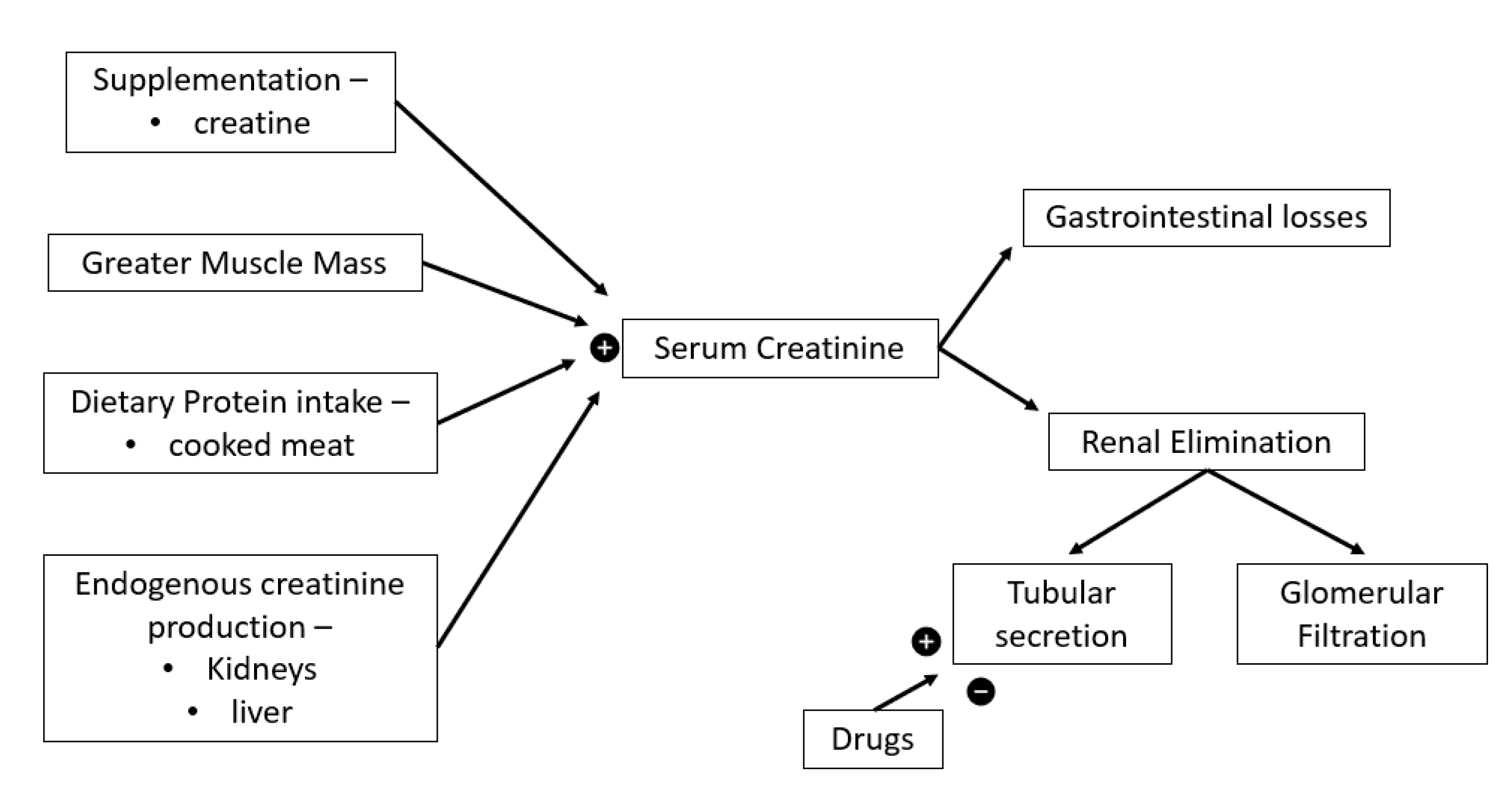
- Kashani, K.; Rosner, M.H.; Ostermann, M. Creatinine: From physiology to clinical application. Eur. J. Int. Med. 2020, 72, 9–14.
- Delanaye, P.; Cavalier, E.; Pottel, H. Serum creatinine: Not so simple! Nephron 2017, 136, 302–308.
- Levey, A.S.; Inker, L.A. Assessment of glomerular filtration rate in health and disease: A state of the art review. Clin. Pharmacol. Ther. 2017, 102, 405–419.
- Botev, R.; Mallié, J.P.; Wetzels, J.F.; Couchoud, C.; Schück, O. The clinician and estimation of glomerular filtration rate by creatinine-based formulas: Current limitations and quo vadis. Clin. J. Am. Soc. Nephrol. 2011, 6, 937–950.
- Kork, F.; Balzer, F.; Krannich, A.; Bernardi, M.H.; Eltzschig, H.K.; Jankowski, J.; Spies, C. Back-calculating baseline creatinine overestimates prevalence of acute kidney injury with poor sensitivity. Acta Physiol. 2017, 219, 615–626.
- Irving, R.A.; Noakes, T.D.; Burger, S.C.; Myburgh, K.H.; Querido, D.; van Zyl Smit, R. Plasma volume and renal function during and after ultramarathon running. Med. Sci. Sports Exerc. 1990, 22, 581–587.
- Poortmans, J.R.; Gulbis, B.; De Bruyn, E.; Baudry, S.; Carpentier, A. Limitations of serum values to estimate glomerular filtration rate during exercise. Br. J. Sports Med. 2013, 47, 1166–1170.
- Little, C.E.; Lipman, G.S.; Migliaccio, D.; Young, D.S.; Krabak, B.J. Accuracy of estimated creatinine in multistage ultramarathon runners. Wilderness Environ. Med. 2019, 30, 129–133.
- Dharnidharka, V.R.; Kwon, C.; Stevens, G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am. J. Kidney Dis. 2002, 40, 221–226.
- Rabb, H.; Griffin, M.D.; McKay, D.B.; Swaminathan, S.; Pickkers, P.; Rosner, M.H.; Kellum, J.A.; Ronco, C. Inflammation in AKI: Current understanding, key questions, and knowledge gaps. J. Am. Soc. Nephrol. 2016, 27, 371–379.
- Tangri, N.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Beck, G.J.; Greene, T.; Coresh, J.; Levey, A.S. Changes in dietary protein intake has no effect on serum cystatin C levels independent of the glomerular filtration rate. Kidney Int. 2011, 79, 471–477.
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29.
- Levey, A.S.; Becker, C.; Inker, L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 2022, 313, 837–846.
- Mingels, A.; Jacobs, L.; Kleijnen, V.; Wodzig, W.; van Dieijen-Visser, M. Cystatin C a marker for renal function after exercise. Int. J. Sports Med. 2009, 30, 668–671.
- Lippi, G.; Sanchis-gomar, F.; Salvagno, G.L.; Aloe, R.; Schena, F.; Guidi, G.C. Variation of serum and urinary neutrophil gelatinase associated lipocalin (NGAL) after strenuous physical exercise. Clin. Chem. Lab. Med. 2012, 50, 1585–1589.
- Zhang, W.R.; Parikh, C.R. Biomarkers of acute and chronic kidney disease. Annu. Rev. Physiol. 2019, 81, 309–333.
- Mishra, J.; Dent, C.; Tarabishi, R.; Mitsnefes, M.M.; Ma, Q.; Kelly, C.; Ruff, S.M.; Zahedi, K.; Shao, M.; Bean, J.; et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005, 365, 1231–1238.
- Wharam, P.C.; Speedy, D.B.; Noakes, T.D.; Thompson, J.M.D.; Reid, S.A.; Holtzhausen, L.-M. NSAID use increases the risk of developing hyponatremia during an Ironman triathlon. Med. Sci. Sports Exerc. 2006, 38, 618–622.
- Whelton, A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: Physiologic foundations and clinical implications. Am. J. Med. 1999, 106, 13S–24S.
- Belli, T.; Macedo, D.V.; De Araujo, G.G.; Dos Reis, I.G.M.; Scariot, P.P.M.; Lazarim, F.L.; Nunes, L.A.S.; Brenzikofer, R.; Gobatto, C.A. Mountain ultramarathon induces early increases of muscle damage, inflammation, and risk for acute renal injury. Front. Physiol. 2018, 9, 1368.
- Murray, M.D.; Brater, D.C. Renal toxicity of the nonsteroidal anti-inflammatory drugs. Annu. Rev. Pharmacol. Toxicol. 1993, 33, 435–465.
- Miller, S.B. Prostaglandins in health and disease: An overview. In Seminars in Arthritis and Rheumatism; WB Saunders: Philadelphia, PA, USA, 2006; Volume 36, pp. 37–49.
- Zyl-smit RVan Mills, P.; Vogelpoel, L. Unrecognised acute renal failure following the comrades marathon. S. Afr. Med. J. 2000, 90, 39–40.
- Shimizu, Y.; Takaori, K.; Maeda, S. Exercise-induced acute renal failure in a trainee cyclist without hypouricemia: Successful athletic career post-treatment. J. Gen. Fam. Med. 2017, 18, 432–435.
- Hoffman, M.D.; Stellingwerff, T.; Costa, R.J. Considerations for ultra-endurance activities: Part 2—Hydration. Res. Sport Med. 2019, 27, 182–194.
- Rojas-Valverde, D.; Sánchez-Ureña, B.; Pino-Ortega, J.; Gómez-Carmona, C.; Gutiérrez-Vargas, R.; Timón, R.; Olcina, G. External workload indicators of muscle and kidney mechanical injury in endurance trail running. Int. J. Environ. Res. Public Health 2019, 16, 3909.
- Knechtle, B.; Nikolaidis, P.T. Physiology and pathophysiology in ultra-marathon running. Front. Physiol. 2018, 9, 634.
- Clarkson, P.M. Exertional rhabdomyolysis and acute renal failure in marathon runners. Sport Med. 2007, 37, 361–363.
- Kim, J.; Lee, J.; Kim, S.; Young, H.; Suk, K. Exercise-induced rhabdomyolysis mechanisms and prevention: A literature review. J. Sport Health Sci. 2016, 5, 324–333.
- Asserraji, M.; Benameur, I.; Maoujoud, O.; El Kharras, A.; Hajbi, H.; Filali, K. Late care in marathon runs leading to exertional heat stroke with multiple organ failure. Asian J. Sports Med. 2014, 5, 136–138.
- Armstrong, L.E. Assessing hydration status: The elusive gold standard. J. Am. Coll. Nutr. 2007, 26, 575–584.
- Goulet, E.D.B. Dehydration and endurance performance in competitive athletes. Nutr. Rev. 2012, 70, S132–S136.
- Cheuvront, S.N.; Carter, R.; Sawka, M.N. Fluid balance and endurance exercise performance. Curr. Sports Med. Rep. 2003, 2, 202–208.
- Hoffman, M.D.; Goulet, E.D.; Maughan, R.J. Considerations in the use of body mass change to estimate change in hydration status during a 161-kilometer ultramarathon running competition. Sport Med. 2018, 48, 243–250.
- Kao, W.F.; Shyu, C.L.; Yang, X.W.; Hsu, T.F.; Chen, J.J.; Kao, W.C.; Huang, Y.J.; Kuo, F.C.; Huang, C.I.; Lee, C.H. Athletic performance and serial weight changes during 12-and 24-hour ultra-marathons. Clin. J. Sport Med. 2008, 18, 155–158.
- King, R.F.G.J.; Jones, B.; Hara, J.P.O. The availability of water associated with glycogen during dehydration: A reservoir or raindrop? Eur. J. Appl. Physiol. 2018, 118, 283–290.
- Hoffman, M.D.; Pasternak, A.; Rogers, I.R.; Khodaee, M.; Hill, J.C.; Townes, D.A.; Scheer, B.V.; Krabak, B.J.; Basset, P.; Lipman, G.S. Medical services at ultra-endurance foot races in remote environments: Medical issues and consensus guidelines. Sport Med. 2014, 44, 1055–1069.
- McDermott, B.P.; Anderson, S.A.; Armstrong, L.E.; Casa, D.J.; Cheuvront, S.N.; Cooper, L.; Kenney, W.L.; O’Connor, F.G.; Roberts, W.O. National athletic trainers’ association position statement: Fluid replacement for the physically active. J. Athl. Train. 2017, 52, 877–895.
- Hoffman, M.D.; Stuempfle, K.J.; Sullivan, K.; Weiss, R.H. Exercise-associated hyponatremia with exertional rhabdomyolysis: Importance of proper treatment. Clin. Nephrol. 2015, 83, 235–242.
- Gill, S.K.; Hankey, J.; Wright, A.; Marczak, S.; Hemming, K.; Allerton, D.M.; Ansley-Robson, P.; Costa, R.J.S. The impact of a 24-h ultra-marathon on circulatory endotoxin and cytokine profile. Int. J. Sports Med. 2015, 36, 688–695.
- Hoffman, M.D.; Weiss, R.H. Does acute kidney injury from an ultramarathon increase the risk for greater subsequent injury? Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2016, 26, 417–422.
- Kupchak, B.R.; Kraemer, W.J.; Hoffman, M.D.; Phinney, S.D.; Volek, J.S. The impact of an ultramarathon on hormonal and biochemical parameters in men. Wilderness Environ. Med. 2014, 25, 278–288.
- Mcvane, B.A.; Andreae, M.C.; Fernando, D.B.; Strayer, R.J. Exertional rhabdomyolysis in a long-distance migrant. J. Emerg. Med. 2019, 56, 551–553.
- Atias-Varon, D.; Sherman, H.; Yanovich, R.; Heled, Y. Rhabdomyolysis after crawling military training. Mil. Med. 2017, 182, e1948–e1952.
- Kim, D.; Ko, E.J.; Cho, H.; Park, S.H.; Lee, S.H.; Cho, N.G.; Lee, S.Y.; Jeong, H.Y.; Yang, D.H. Spinning-induced rhabdomyolysis: Eleven case reports and review of the literature. Electrolytes Blood Press. 2015, 13, 58–61.
- Oh, R.C.; Arter, J.L.; Tiglao, S.M.; Larson, S.L. Exertional rhabdomyolysis: A case series of 30 hospitalized patients. Mil. Med. 2015, 180, 201–207.
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767.
- Stahl, K.; Rastelli, E.; Schoser, B. A systematic review on the definition of rhabdomyolysis. J. Neurol. 2020, 267, 877–882.
- Tietze, D.C.; Borchers, J. Exertional rhabdomyolysis in the athlete: A clinical review. Sports Health 2014, 6, 336–339.
