Cancer is one of the longest-known human diseases, known at least from ancient Egyptian papyri. Even though the causal association between cancer and occupational exposure to pollutants can be inferred from the works of Paracelsus, only in the early 20th century onward, when the nature and role of DNA was unravelled, could oncobiologists and toxicologists join efforts to endeavour understanding mechanism and risk. Nowadays weit is known that chemically-induced cancers of environmental origin (excluding tobacco smoking) can represent about 10% or more of the total number of incidences, globally. The paradigmatic case of asbestos in the second half of the 20th century was arguably the first incident to increase the awareness for environmental carcinogens in a global scale. However, it resulted in a long and painstaking ban process that altogether highlights the challenges of safeguarding human and environmental health.
- neoplasia
- cancer
- pollution
- contamination
- genotoxicants
1. Introduction
2. A Brief Historical Overview of Cancer and Pollution
The great scientific gap of the Dark Ages brought biomedical sciences in western civilisation to a stand-still. However, Arabic physicians like Avicenna (980–1037) and philosophers like Averroes (1126–1198) collected, expanded and taught scientific knowledge, essentially keeping alive the flame of early biomedicine in times of strict Christian fundamentalism and feudal obscurantism. Their work and teachings were fundamental pillars upon which European medicine would be rebuilt during the Renaissance. The Swiss-German physician and alchemist Theophrast von Hohenheim (1493–1541), better known as Paracelsus, who is considered the founder of modern toxicology by first acknowledging dose-effect relationships (‘it is only the dose which separates benefit from poison’), was seemingly the first to draw conclusions on chemical-induced carcinogenesis. In his posthumously published work on the ‘mountain disease’ of miners (1567), Paracelsus already linked illnesses such as tuberculosis and lung cancer to exposure to ‘poisonous air’. This work (originally named Von der Bergsucht und anderen Bergkrankheiten) was born from Paracelsus’ direct observations of miners and their environment. It can very well be the first occurrence of what we would nowadays call an association between occupational exposure and cancer—in a time were diseases were mostly attributed to mystical causes (the reader is diverted to the review by Hayes and Gilbert [21] on the hallmarks of toxicology). Additionally, the Italian physician Bernardino Ramazzini (1633–1714), often regarded to be the founder of occupational medicine, in his most famous book, De Morbis Artificum Diatriba (‘On the Diseases of Workers’), already discussed the environment-related aetiology of various diseases, from pneumoconiosis to breast cancer (see Franco [22]). It needs to be noted, though, that if Theophrast established a link between toxicology and disease and Ramazzini associated occupational exposure to disease, including cancer, the study of chemical toxicology at this stage was still struggling with establishing causation due to the lack of identification of carcinogens per se. Nonetheless, despite rudimentary or a priori absent tools, The Illuminist Man would slowly, but steadily, find his course (see Figure 1 for a historical overview).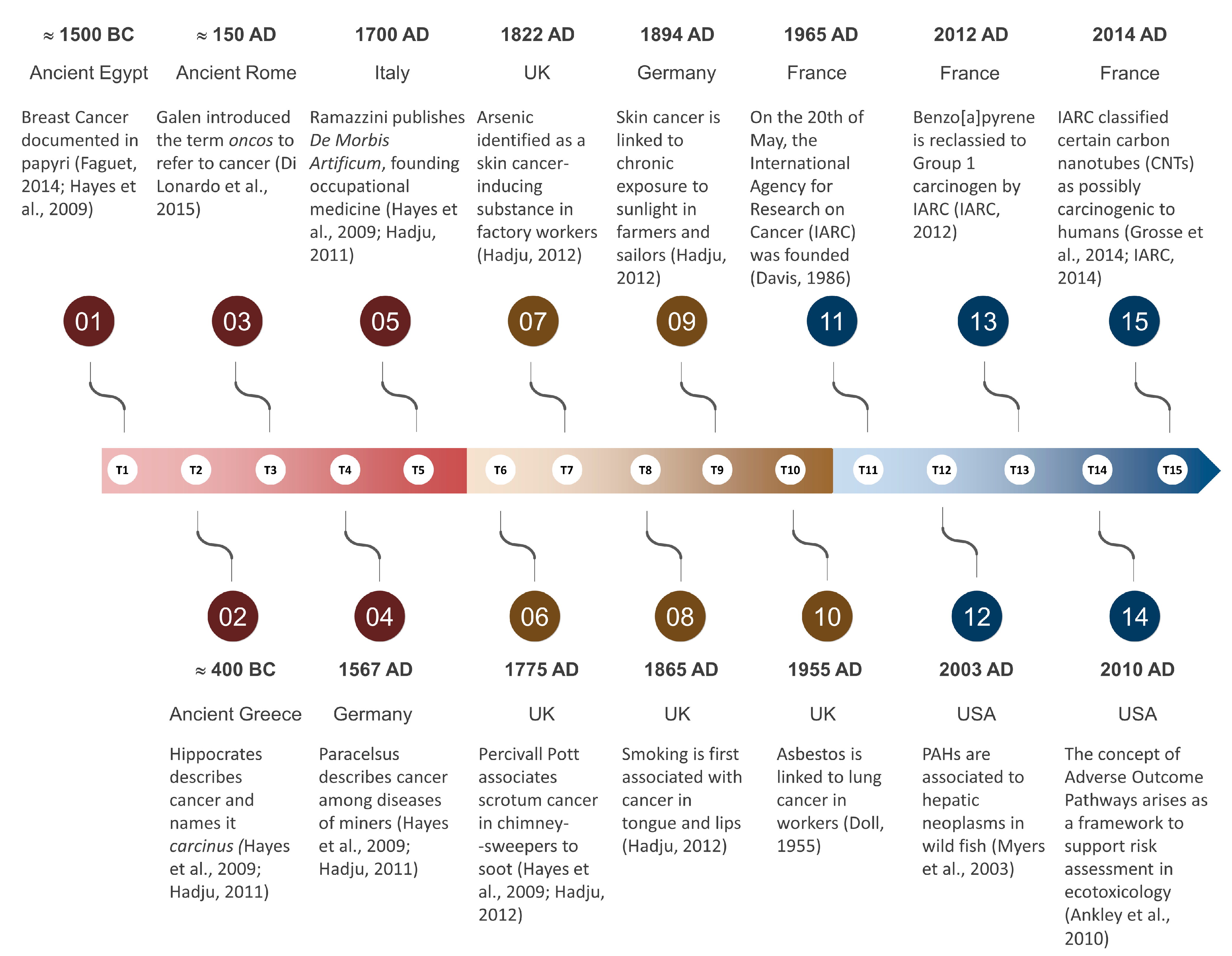
References
- Sullivan, R. The identity and work of the ancient Egyptian surgeon. J. R. Soc. Med. 1996, 89, 467–473.
- Faguet, G.B. A brief history of cancer: Age-old milestones underlying our current knowledge database. Int. J. Cancer 2014, 136, 2022–2036.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Colditz, G.A.; Wei, E.K. Preventability of cancer: The relative contributions of biologic and social and physical environmental determinants of cancer mortality. Annu. Rev. Public Health 2012, 33, 137–156.
- Madia, F.; Worth, A.; Whelan, M.; Corvi, R. Carcinogenicity assessment: Addressing the challenges of cancer and chemicals in the environment. Environ. Int. 2019, 128, 417–429.
- Rothschild, B.M.; Tanke, D.H.; Helbling, M., II; Martin, L.D. Epidemiologic study of tumors in dinosaurs. Naturwissenschaften 2003, 90, 495–500.
- Avery, O.T.; MacLeod, C.M.; McCarty, M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a desoxyribosenucleic acid fraction isolated from Pneumococcus type III. J. Exp. Med. 1944, 79, 137–158.
- Franklin, R.E.; Gosling, R.G. The structure of sodium thymonucleate fibres. I. The influence of water content. Acta Crystallogr. 1953, 6, 673–677.
- Watson, J.D.; Crick, F.H.C. Molecular structure of nucleic acids: A structure for deoxyribose nucleic acid. Nature 1953, 171, 737–738.
- Shih, C.; Weinberg, R.A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell 1982, 29, 161–169.
- Duesberg, P.H.; Vogt, P.K. Differences between the ribonucleic acids of transforming and nontransforming avian tumor vi-ruses. Proc. Natl. Acad. Sci. USA 1982, 67, 1673–1680.
- Reddy, E.P.; Reynolds, R.K.; Santos, E.; Barbacid, M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature 1982, 300, 149–152.
- Costa, P.M. Current aspects of DNA damage and repair in ecotoxicology: A mini-review. Ecotoxicology 2022, 31, 1–11.
- Ross, J.; Nesnow, S. Polycyclic aromatic hydrocarbons: Correlations between DNA adducts and ras oncogene mutations. Mutat. Res. Mol. Mech. Mutagen. 1999, 424, 155–166.
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic aromatic hydrocarbons: From metabolism to lung cancer. Toxicol. Sci. 2015, 145, 5–15.
- DeMarini, D.M.; Linak, W.P. Mutagenicity and carcinogenicity of combustion emissions are impacted more by combustor technology than by fuel composition: A brief review. Environ. Mol. Mutagen. 2022, 63, 135–150.
- Nelson, D.R.; Goldstone, J.V.; Stegeman, J.J. The cytochrome P450 genesis locus: The origin and evolution of animal cytochrome P450s. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120474.
- Kawashima, A.; Satta, Y. Substrate-dependent evolution of cytochrome P450: Rapid turnover of the detoxification-type and conservation of the biosynthesis-type. PLoS ONE 2014, 9, e100059.
- Ankley, G.T.; Bennett, R.S.; Erickson, R.J.; Hoff, D.J.; Hornung, M.W.; Johnson, R.D.; Mount, D.R.; Nichols, J.W.; Russom, C.L.; Schmieder, P.K.; et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010, 29, 730–741.
- Martins, C.; Dreij, K.; Costa, P.M. The state-of-the art of environmental toxicogenomics: Challenges and perspectives of “omics” approaches directed to toxicant mixtures. Int. J. Environ. Res. Public Health 2019, 16, 4718.
- Hayes, A.N.; Gilbert, S.G. Historical milestones and discoveries that shaped the toxicology sciences. EXS 2009, 99, 1–35.
- Franco, G. Bernardino Ramazzini’s De Morbis Artificum Diatriba on workers’ health–the birth of a new discipline. J. UOEH 2021, 43, 341–348.
- Hajdu, S.I. A note from history: Landmarks in history of cancer, part 1. Cancer 2011, 117, 1097–1102.
- Di Lonardo, A.; Nasi, S.; Pulciani, S. Cancer: We should not forget the past. J. Cancer 2015, 6, 29–39.
- Hajdu, S.I. A note from history: Landmarks in history of cancer, part 2. Cancer 2011, 117, 2811–2820.
- Hajdu, S.I. A note from history: Landmarks in history of cancer, part 3. Cancer 2012, 118, 1155–1168.
- Doll, R. Mortality from Lung Cancer in Asbestos Workers. Occup. Environ. Med. 1955, 12, 81–86.
- Davis, W. IARC: 20 Years Old. World Health 1986, 1986, 28–29.
- Myers, M.S.; Johnson, L.L.; Collier, T.K. Establishing the causal relationship between polycyclic aromatic hydrocarbon (PAH) exposure and hepatic neoplasms and neoplasia-related liver lesions in English sole (Pleuronectes vetulus). Hum. Ecol. Risk Assessment Int. J. 2003, 9, 67–94.
- IARC. Benzopyrene. In IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 100F.
- Grosse, Y.; Loomis, D.; Guyton, K.Z.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Scoc-cianti, C.; Mattock, H.; et al. Carcinogenicity of fluoro-edenite, silicon carbide fibres and whiskers, and carbon nanotubes. Lancet Oncol. 2014, 15, 1427–1428.
- IARC. Carbon nanotubes. In IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans; IARC: Lyon, France, 2014; Volume 111.
- Kennaway, E.L. On the cancer-producing factor in tar. Br. Med. J. 1924, 1, 564–567.
- Yamagiwa, K.; Ichikawa, K. Über die künstliche Erzeugung von Papillom. Verh. Jap. Path. 1915, 5, 142–148.
- Phillips, D.H. Fifty years of benzo(a)pyrene. Nature 1983, 303, 468–472.
- Fujiki, H. Gist of Dr. Katsusaburo Yamagiwa’s papers entitled “Experimental study on the pathogenesis of epithelial tumors” (I to VI reports). Cancer Sci. 2014, 105, 143–149.
- Boveri, T. Concerning the Origin of Malignant Tumours by Theodor Boveri. Translated and annotated by Henry Harris. J. Cell Sci. 2008, 121, 1–84.
- Bolognesi, C.; Perrone, E.; Roggieri, P.; Pampanin, D.M.; Sciutto, A. Assessment of micronuclei induction in peripheral eryth-rocytes of fish exposed to xenobiotics under controlled conditions. Aquat. Toxicol. 2006, 78, S93–S98.
- Costa, P.M.; Costa, M.H. Genotoxicity assessment in fish peripheral blood: A method for a more efficient analysis of micronuclei. J. Fish Biol. 2007, 71, 148–151.
- Fenech, M.; Kirsch-Volders, M.; Natarajan, A.T.; Surralles, J.; Crott, J.W.; Parry, J.; Norppa, H.; Eastmond, D.A.; Tucker, J.D.; Thomas, P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 2010, 26, 125–132.
- Singh, N.P.; Mccoy, M.T.; Tice, R.R.; Scineider, E.L. A simple technique for quantification of low levels of DNA in individual cells. Exp. Cell. Res. 1988, 175, 184–191.
- Evans, H.; Neary, G.; Williamson, F. The relative biological efficiency of single doses of fast neutrons and gamma-rays on Vicia faba roots and the effect of oxygen. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1959, 1, 216–229.
- Muller, H.J. Artificial transmutation of the gene. Science 1927, 66, 84–87.
- Gunderman, R.B.; Gonda, A.S. Radium girls. Radiology 2015, 274, 314–318.
- Carson, R. Silent Spring; Houghton Mifflin: Boston, MA, USA, 1962.
- Patterson, C.C. Contaminated and natural lead environments of Man. Arch. Environ. Health Int. J. 1965, 11, 344–360.
- IARC. Inorganic and Organic Lead Compounds. In IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans; IARC: Lyon, France, 2006; Volume 87.
- IARC. DDT, Lindane, and 2,4-D. In IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans; IARC: Lyon, France, 2018; Volume 113.
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58.
