Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Zoe Price.
Disabled-2 (DAB2), a key adaptor protein in clathrin mediated endocytosis, is implicated in the regulation of key signalling pathways involved in homeostasis, cell positioning and epithelial to mesenchymal transition (EMT).
- DAB2
- cancer
- metastasis
1. Introduction
Disabled-2 (DAB2) is a widely recognised tumour suppressor. It was initially discovered in 1994 when Mok et al. identified an 800bp cDNA fragment which was expressed in normal ovarian surface epithelial cell lines but not in ovarian cancer cell lines. They referred to it as differentially expressed in ovarian carcinoma 2 (DOC-2) [1]. The following year, Xu et al. identified a 96 kDa phosphoprotein in mouse macrophage cell line, BAC1.2F5 with an amino terminal end which shared homology to the Drosophila disabled gene [2,3][2][3]. The Drosophila disabled protein is important in embryogenesis and neural positioning [4]. DAB2 is one of two human orthologs of the Drosophila disabled gene, Disabled-1 is expressed almost exclusively in neural cells whereas DAB2 is expressed in a wide range of epithelial cells including those of the ovary, lung and breast [5,6,7][5][6][7]. Loss of DAB2 expression has been reported in a range of malignancies including ovarian, lung and breast cancer [5,6,7][5][6][7]. The loss of DAB2 is associated with activation of key signalling pathways including wingless/integrated (Wnt, MAPK and TGFβ), mitogen activated protein kinase (MAPK) and transforming growth factor beta (TGFβ) which is associated with enhanced cell proliferation, chemotherapy resistance and tumour progression, supporting its role as a tumour suppressor.
2. DAB2 Structure and Function
The human DAB2 gene, located on chromosome 5p13 consists of 15 exons, encoding a 770 amino acid protein [8]. The mouse DAB2 gene has 83% homology with the human gene, it also consists of 15 exons and encodes a 766 amino acid protein [9]. There are two isoforms of DAB2, including full length p96 (also known as p82) and spliced p67 (also known as p59) that is missing the central exon. DAB2 contains binding domains and motifs which allow it to recognise and recruit proteins to clathrin coated pits for endocytosis (Figure 1). Two key binding domains of DAB2 are a phosphotyrosine binding (PTB) domain at the N-terminus and a proline rich domain (PRD) at the carboxy terminal end of the protein which contains a myosin interacting region (MIR). The main function of DAB2 is as a clathrin associated sorting protein (CLASP) in clathrin mediated endocytosis. DAB2 interacts with clathrin via multiple binding sites including a type I LVDLN and type II PWPYP sequence [10]. DAB2 can interact with both pre-assembled clathrin cages and also soluble clathrin trimers, indicating a possible role in clathrin cage assembly [10]. The DAB2 PTB binds to phosphoinositide(4,5)P2 (PtdIns(4,5)P2) containing liposomes, further suggesting it is involved in clathrin cage assembly and vesicle budding [10].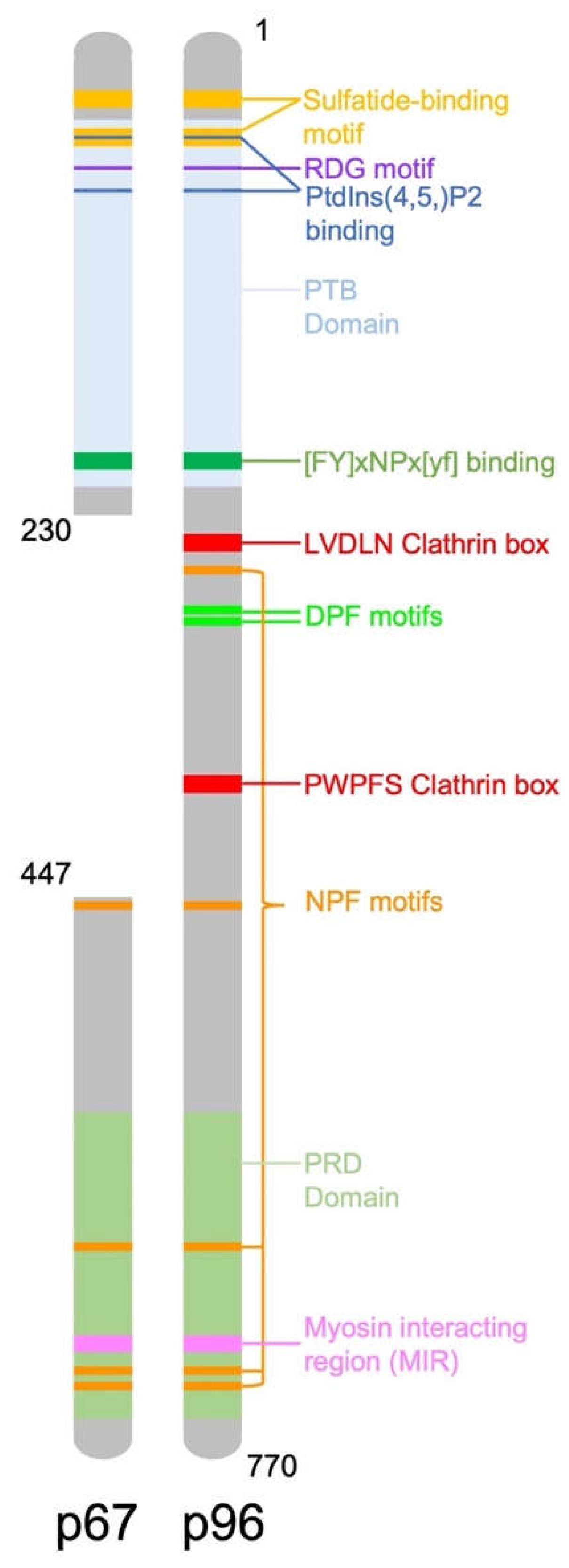
Figure 1.
Diagram representing the structure of the DAB2 protein and location of important functional domains.
References
- Mok, S.C.; Wong, K.K.; Chan, R.K.; Lau, C.C.; Tsao, S.W.; Knapp, R.C.; Berkowitz, R.S. Molecular cloning of differentially expressed genes in human epithelial ovarian cancer. Gynecol. Oncol. 1994, 52, 247–252.
- Xu, X.X.; Yi, T.; Tang, B.; Lambeth, J.D. Disabled-2 (DAB2) is an SH3 domain-binding partner of Grb2. Oncogene 1998, 16, 1561–1569.
- Xu, X.X.; Yang, W.; Jackowski, S.; Rock, C.O. Cloning of a novel phosphoprotein regulated by colony-stimulating factor 1 shares a domain with the Drosophila disabled gene product. J. Biol. Chem. 1995, 270, 14184–14191.
- Gertler, F.B.; Bennett, R.L.; Clark, M.J.; Hoffmann, F.M. Drosophila abl tyrosine kinase in embryonic CNS axons: A role in axonogenesis is revealed through dosage-sensitive interactions with disabled. Cell 1989, 58, 103–113.
- Bagadi, S.A.; Prasad, C.P.; Srivastava, A.; Prashad, R.; Gupta, S.D.; Ralhan, R. Frequent loss of DAB2 protein and infrequent promoter hypermethylation in breast cancer. Breast Cancer Res. Treat. 2007, 104, 277–286.
- Sheng, Z.; Sun, W.; Smith, E.; Cohen, C.; Sheng, Z.; Xu, X.X. Restoration of positioning control following Disabled-2 expression in ovarian and breast tumor cells. Oncogene 2000, 19, 4847–4854.
- Xu, H.T.; Yang, L.H.; Li, Q.C.; Liu, S.L.; Liu, D.; Xie, X.M.; Wang, E.H. Disabled-2 and Axin are concurrently colocalized and underexpressed in lung cancers. Hum. Pathol. 2011, 42, 1491–1498.
- Sheng, Z.; He, J.; Tuppen, J.A.; Sun, W.; Fazili, Z.; Smith, E.R.; Dong, F.B.; Xu, X.X. Structure, sequence, and promoter analysis of human disabled-2 gene (DAB2). Genomics 2000, 70, 381–386.
- Albertsen, H.M.; Smith, S.A.; Melis, R.; Williams, B.; Holik, P.; Stevens, J.; White, R. Sequence, genomic structure, and chromosomal assignment of human DOC-2. Genomics 1996, 33, 207–213.
- Mishra, S.K.; Keyel, P.A.; Hawryluk, M.J.; Agostinelli, N.R.; Watkins, S.C.; Traub, L.M. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 2002, 21, 4915–4926.
- Morris, S.M.; Cooper, J.A. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic 2001, 2, 111–123.
- Maurer, M.E.; Cooper, J.A. The adaptor protein DAB2 sorts LDL receptors into coated pits independently of AP-2 and ARH. J Cell Sci. 2006, 119, 4235–4246.
- Yu, C.; Feng, W.; Wei, Z.Y.; Miyanoiri, Y.; Wen, W.Y.; Zhao, Y.X.; Zhang, M.J. Myosin VI Undergoes Cargo-Mediated Dimerization. Cell 2009, 138, 537–548.
- Rai, A.; Vang, D.; Ritt, M.; Sivaramakrishnan, S. Dynamic multimerization of DAB2-Myosin VI complexes regulates cargo processivity while minimizing cortical actin reorganization. J. Biol. Chem. 2021, 296, 100232:1–100232:13.
- Morris, S.M.; Arden, S.D.; Roberts, R.C.; Kendrick-Jones, J.; Cooper, J.A.; Luzio, J.P.; Buss, F. Myosin VI binds to and localises with DAB2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic 2002, 3, 331–341.
- Fili, N.; Hari-Gupta, Y.; Aston, B.; dos Santos, A.; Gough, R.E.; Alamad, B.; Wang, L.; Martin-Fernandez, M.L.; Toseland, C.P. Competition between two high-and low-affinity protein-binding sites in myosin VI controls its cellular function. J. Biol. Chem. 2020, 295, 337–347.
- Da Paz, V.F.; Ghishan, F.K.; Kiela, P.R. Emerging Roles of Disabled Homolog 2 (DAB2) in Immune Regulation. Front. Immunol. 2020, 11, 580302:1–580302:11.
- Rosenbauer, F.; Kallies, A.; Scheller, M.; Knobeloch, K.P.; Rock, C.O.; Schwieger, M.; Stocking, C.; Horak, I. Disabled-2 is transcriptionally regulated by ICSBP and augments macrophage spreading and adhesion. Embo J. 2002, 21, 211–220.
- Adamson, S.E.; Griffiths, R.; Moravec, R.; Senthivinayagam, S.; Montgomery, G.; Chen, W.; Han, J.; Sharma, P.R.; Mullins, G.R.; Gorski, S.A.; et al. Disabled homolog 2 controls macrophage phenotypic polarization and adipose tissue inflammation. J. Clin. Investig. 2016, 126, 1311–1322.
- Jokubaitis, V.G.; Gresle, M.M.; Kemper, D.A.; Doherty, W.; Perreau, V.M.; Cipriani, T.L.; Jonas, A.; Shaw, G.; Kuhlmann, T.; Kilpatrick, T.J.; et al. Endogenously regulated DAB2 worsens inflammatory injury in experimental autoimmune encephalomyelitis. Acta Neuropathol. Commun. 2013, 1, 32:1–32:14.
- Figliuolo da Paz, V.; Jamwal, D.R.; Gurney, M.; Midura-Kiela, M.; Harrison, C.A.; Cox, C.; Wilson, J.M.; Ghishan, F.K.; Kiela, P.R. Rapid Downregulation of DAB2 by Toll-Like Receptor Activation Contributes to a Pro-Inflammatory Switch in Activated Dendritic Cells. Front. Immunol. 2019, 10, 304:1–304:18.
- Lin, W.; Wang, W.; Wang, D.; Ling, W. Quercetin protects against atherosclerosis by inhibiting dendritic cell activation. Mol. Nutr. Food Res. 2017, 61, 1700031:1–1700031:12.
- Jain, N.; Nguyen, H.; Friedline, R.H.; Malhotra, N.; Brehm, M.; Koyanagi, M.; Bix, M.; Cooper, J.A.; Chambers, C.A.; Kang, J. Cutting edge: DAB2 is a FOXP3 target gene required for regulatory T cell function. J. Immunol. 2009, 183, 4192–4196.
More
