Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Gastón Fuentes and Version 2 by Conner Chen.
Cancer has become one of the deadliest diseases in our society. Surgery accompanied by subsequent chemotherapy is the treatment most used to prolong or save the patient’s life. Still, it carries secondary risks such as infections and thrombosis and causes cytotoxic effects in healthy tissues. Using nanocarriers such as smart polymer micelles is a promising alternative to avoid or minimize these problems. These nanostructured systems will be able to encapsulate hydrophilic and hydrophobic drugs through modified copolymers with various functional groups such as carboxyls, amines, hydroxyls, etc.
- smart polymeric micelles
- anticancer hydrophobic drugs
- nanocarriers
1. Introduction
Cancer is one of the leading causes of death worldwide. Surgery accompanied by subsequent chemotherapy is the most widely used treatment to lengthen or save the patient’s life. Surgery inherently bears secondary risks, such as infection and thrombosis. Chemotherapeutic compounds may be ineffective due to low water solubility, low tumor targeting, and low cellular uptake, and they may cause cytotoxic effects on healthy tissues [1]. In cancer treatment, nanocarrier systems have great potential to help or replace traditional chemotherapy. Controlled drug delivery by nanoparticles is crucial but has always been a significant challenge in developing new effective cancer therapies and diagnostics. The nanocarriers most used for these purposes are carbon nanotubes [2], dendrimer [3], micelles [4], quantum dot [5], liposomes [6], cubosomes [7], magnetic nanoparticles [8], gold nanoparticles, and mesoporous silica nanoparticles (Figure 1) [9][10][9,10].
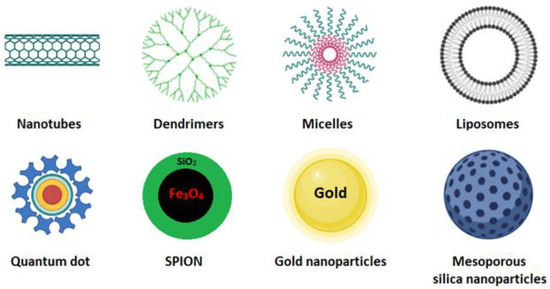
Figure 1.
These systems, also known as smart drug-delivery systems (SDDS), are developed so that affected cells, but not healthy cells, locally deliver therapeutic agents at the right concentration. The release of these agents occurs by physicochemical mechanisms that depend on external or internal stimuli that act on the nanoparticles [11][12][11,12]. These stimuli include pH gradients [13], redox reactions [14], enzymatic cleaving [15], temperature [16][17][18][16,17,18], ultrasound waves [19], light [20], and magnetic and electric fields [8][21][8,21].
All these nanocarriers, in one way or another, always contain a hydrophilic polymer to (i) improve their solubility or stability in extracellular fluids, (ii) avoid processes of exclusion by the immune system and rapid elimination by the reticuloendothelial system, and (iii) functionalize molecules (peptides, proteins, antibodies, genetic material, etc.) that allow the drug to be guided to the target and to be released. This conjugation is always done by forming amide, disulfide, or ester bonds [10]. Within SDSS, polymeric micelles have gained renewed interest due to their physicochemical properties that optimize accumulation in tissues caused by the increased vascular permeability in sites of cancer or inflammation, the so-called enhanced permeation, and the retention (EPR) effect that regulates tumor targeting. Furthermore, polymeric micelles notably increase the solubility of hydrophobic drugs in water by 10- to 5000-fold. The drugs are stabilized in a core composed of a hydrophobic polymeric or lipid matrix covered by a hydrophilic polymer. This modification will prolong the circulation of drugs in the bloodstream. The synthesis of micelles as an SDDS constitutes a potential research topic because of the wide variety of biocompatible and biodegradable polymers and lipids [22][23][22,23].
2. Polymeric Micelles
Polymeric micelles are formulated by self-assembly in an aqueous medium of block copolymers that spontaneously form a core–shell structure with a marked amphiphilic character. The differences between the hydrophobic and hydrophilic segments will directly influence the micellization process for forming this type of nanostructures. Copolymers are classified according to the number of monomers in their structure, such as di-, tri-, tetra-, and multiblock copolymers. Polymeric micelles generally have a hydrophobic core with a hydrophilic coating. Most therapeutic cancer agents are hydrophobic and can therefore be encapsulated in the core. These copolymers can be modified unlimitedly, making them either more hydrophobic or hydrophilic, depending on the chemical properties of the drug under study. In this way, the stability and solubility of these drugs in biological systems can be improved [24]. Figure 2 shows the most frequently used polymers as a hydrophobic core.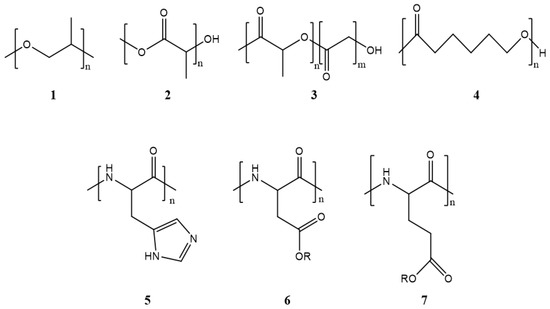
Figure 2. Structure of the most frequently used polymers as a core in the micelle’s preparation. Polyethers; 1: poly(propylene oxide) (PPO) [25]. Polyesters; 2: poly(L-lactide) (PLA) [26], 3: poly(lactide-co-glycolide) (PLGA), 4: poly(ε-caprolactone) (PCL) [27], 5: poly(L-histidine)(pHis) [28], 6: poly(L-aspartic acid) and derivatives (pAsp) [29], 7: poly(L-glutamic acid) and derivatives (pGlu) [30].
ΔG0 = RT ln(CMC)
3. Micelle Synthesis
Five techniques are used to synthesize micelles. These are (1) direct solution, (2) oil in water emulsification, (3) thin-film hydration/solvent evaporation (4) dialysis, and (5) freeze-drying (Table 1). The applied technique depends on the solubility of the selected copolymer. The polymeric micelle is classified as regular or direct if the copolymer’s hydrophilic part is exposed on its surface. On the other hand, the micelles are called inverse when they do not have a copolymer with the hydrophilic part exposed on the surface [10].Table 1.
| Methods | Advantage/Disadvantage | Drug-Loading Capacity | Solvents | Types of Drugs | Encapsulated Anticancer Drug | Polymers Used |
|---|---|---|---|---|---|---|
| Direct dissolution | The simplest technique to prepare polymeric micelles. Does not use organic solvents. Low-molecular-weight hydrophilic polymers | Low drug-loading capacity due to water solubility of polymers | Water | Not applicable for most hydrophobic drugs | Paclitaxel [41] | Mostly hydrophilic polymers; PLA-PEG |
| Docetaxel [42] | d-a-tocopheryl PEG1000 succinate (TPGS) | |||||
| Doxorubicin [43] | Pluronic F127/poly (methyl vinyl ether-alt-maleic acid) | |||||
| Oil-in-water emulsification | Easy preparation. Small particles with a narrow size distribution. Not environmentally friendly due to the use of chlorinated organic solvents. | High drug-loading capacity | Organic solvents immiscible in water (CHCl3, EtAc, and CH2Cl2) | Hydrophobic drugs | Doxorubicin and erlotinib [44] | PLGA/pluronic F-127 |
| Triptorelin [45] | PLA/PLGA | |||||
| Thin-film hydration/solvent evaporation | Only applicable for copolymers with high hydrophilic–lipophilic balance (HLB). Feasible for scaling up but very expensive | High drug-loading capacity and encapsulation efficiency | Water-miscible volatile organic solvents (DMF, THF, DMSO, acetonitrile, MeOH, acetone) | Hydrophobic drugs | Doxorubicin [46] | PEG 5000-lysine-di-tocopherol succinate (P5kSSLV) |
| Curcumin [47] | Poly(ethyleneoxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO-b-PPO-b-PEO/pluronic F-127) | |||||
| Paclitaxel [48] | Inutec SP11 (INT) | |||||
| Dialysis | For highly hydrophobic polymers with long alkyl chains. Difficulty releasing. Easy to remove organic solvents. Not applicable on a large scale due to high water consumption. | High drug-loading capacity | Water-miscible volatile organic solvents (DMF, THF, DMSO, acetonitrile, MeOH, acetone) | Hydrophobic drugs | Docetaxel [49] | PEG/hyperbranched poly(amidoamine) HAPH |
| Docetaxel [50] | PLGA/PEG–maleimide | |||||
| Doxorubicin [51] | PCL-S-S- biodegradable photoluminescent polymer (BPLP) | |||||
| Freeze-drying | High stability and narrow size distribution. Organic-solvent reusability. Thermolabile drug-encapsulation suitability. Limited lyophilize organic solvents and copolymers soluble in them. | High drug-loading capacity | The mixture of water and freeze-dryable organic solvents such as tert-butanol and dimethyl acetamide | Hydrophobic drugs | TM-2 [52] | mPEG/PLA |
| Docetaxel [53] | Thermosensitive methoxy poly(ethylene glycol)-b-poly[N-(2-hydroxypropyl) methacrylamide lactate] (mPEG-bpHPMAmLacn) |