Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Mahesh Chathuranga Abraham Galappaththi and Version 3 by Rita Xu.
Ganoderma has been used as a traditional medicine in Asian countries to prevent and treat various diseases. Numerous publications are stating that Ganoderma species have a variety of beneficial medicinal properties, and investigations on different metabolic regulations of Ganoderma species, extracts or isolated compounds have been performed both in vitro and in vivo.
- bioactivity
- bioactive molecules
- drug
- Ganoderma
- Triterpenoids
1. Introduction
Ganoderma P. Karst. 1881 [1] is an old polypore genus typified by Ganoderma lucidum (Curtis) P. Karst. belonging to the family Ganodermataceae (basidiomycete) [2], which has been synonymized with the family Polyporaceae [3], with the members normally growing on woody plants and logs [2][4][5][2,4,5]. The genus was originally reported in the UK [6] and is considered to have a worldwide distribution [7][8][9][10][11][12][13][14][15][16][17][18][7,8,9,10,11,12,13,14,15,16,17,18]. Ganoderma species are used for medicinal purposes in China [19][20][21][22][19,20,21,22], Japan, South Korea [22] and the French West Indies [12]. The common names for Ganoderma include Lingzhi, Munnertake, Sachitake, Reishi, and Youngzhi. Ganoderma was first recorded in Shennong’s Classic of Materia Medica and classified as an upper-grade medicine in medical books [23]. Index Fungorum (2022) (http://www.indexfungorum.org/, accessed on 6 September 2022) lists 488 records of Ganoderma while MycoBank lists 529 records [18]. Approximately 80 species of Ganoderma are recorded in Chinese Fungi [24], of which G. lucidum and G. sinense are described as medicinally beneficial macrofungi in Chinese medicine [25]. However, other species, such as G. capense, G. cochlear and G. tsugae also play an important role in traditional folk medicine. In addition, pharmacological studies have also used the extract and chemical constituents of other Ganoderma species [22][26][27][28][29][30][22,26,27,28,29,30].
The chemical constituents and the biological activities of 25 species of Ganoderma, namely G. amboinense, G. annulare, G. applanatum (synonym G. lipsiense), G. australe, G. boninense, G. capense, G. carnosum, G. casuarinicola, G. cochlear, G. colossus, G. concinnum, G. ellipsoideum, G. fornicatum, G. hainanense, G. lucidum, G. mastoporum, G. neo-japonicum, G. orbiforme, G. pfeifferi, G. resinaceum, G. sinense, G. theaecola (as theaecolum), G. tropicum, G. tsugae and G. weberianum were studied and reported in this review. Several chemical constituents such as ganoderic acids, lucidenic acids, 12-hydroxyganoderic acid, ganorbiformin, lucidimines [31] and other compounds have been reported from various Ganoderma species during recent decades [29][32][33][34][35][29,32,33,34,35].
Triterpenes, polysaccharides and peptidoglycans are one of the major types of bioactive substances [36][37][36,37] responsible for the various biological activities of several species in Ganoderma (e.g., Ganoderma lucidum). Triterpenoids and ganoderic acids that play a critical role in pharmacological activities are also present in G. applanatum and G. orbiforme [33][36][38][39][40][41][42][33,36,38,39,40,41,42]. Besides G. applanatum [43][44][43,44], G. colossus [45] and G. pfeifferi [46], G. resinaceum [45] have also been identified as potential Ganoderma natural antioxidants.
G. amboinense [47], G. annulare [48], G. applanatum [49][50][51][49,50,51], G. boninense [52], G. calidophilum [53], G. capense [54], G. carnosum [55], G. cochlear [56][57][58][56,57,58], G. colossum [59], G. concinnum, G. neo-japonicum [60], G. fornicatum [61], G. hainanense [62], G. leucocontextum [63], G. lucidum [64][65][66][67][64,65,66,67], G. mastoporum [68], G. mbrekobenum [69], G. resinaceum [70], G. sinense [71][72][73][71,72,73], G. theaecola (as theaecolum) [74], G. tropicum [75], G. tsugae [76], G. weberianum [77] were reported to contain bioactive compounds of great interest.
The members of the genus Ganoderma are rich in novel “mycochemicals”, including polysaccharides [78], steroids, fatty acids, phenolic compounds, vitamins, amino acids and triterpenoids [69][79][80][81][69,79,80,81]. Ganoderma polysaccharides are a hot topic in the medicinal mushroom research field [78][82][83][84][78,82,83,84]. Although polysaccharides are found to be one of the main bioactive constituents, their high molecular weight and complex structure limit their use in the drug market [85]. The Ganoderma polysaccharides are well known for their diverse bioactivities such as antitumor, immunomodulatory, anti-hypertensive, antidyslipidemic and hepatoprotective activities. Ganoderma polysaccharides have been developed into medicines, dietary supplements, healthy foods, treat and prevent diseases, and are continuing to serve as important leads in modern drug discovery [78][86][87][88][78,86,87,88].
Taxonomic Studies of Ganoderma
The word Ganoderma is derived from the Greek words “Gano”, meaning “shiny”, and “derma”, meaning “skin” [22][89][22,101]. Originating from the tropics and recently extending its range into temperate zones [90][102], Ganoderma is an old genus with a taxonomic history dating back to 1881, and the Finnish mycologist P. A. Karsten erected the genus to place a single species, Polyporus lucidus [=Ganoderma lucidum (Curtis: Fr.) P. Karst.] [1][91][1,103]. Ganoderma lucidum is the type species of the genus Ganoderma, which was originally described as from the UK and later found to have a worldwide distribution [6]. A number of Ganoderma species morphologically closely related and belonging to G. lucidum complex viz. G. multipileum Ding Hou 1950 [92][104], G. sichuanense J.D. Zhao & X.Q. Zhang 1983 [93][105] and G. lingzhi Sheng H. [94][95][106,107] from China, G. resinaceum Boud. 1889 [96][108] from Europe, and G. oregonense Murrill 1908, G. sessile Murrill 1902, G. tsugae Murrill 1902 and G. zonatum Murrill 1902 [97][98][109,110] from the USA, have been described as from all over the world, and are mainly characterized by laccate pileus. Zhou et al. [99][111] considered 32 collections belonging to the G. lucidum complex from Asia, Europe and North America in terms of their morphology and phylogeny as derived from analyses of four protein-coding genes viz. Internal transcribed spacer (ITS), translational elongation factor 1-α (tef1-α) and retinol-binding protein 1 and 2 (rpb1, rpb2). Different molecular techniques have been used to study the genetic diversity in Ganoderma, such as amplified fragment length polymorphism, isozyme analysis, inter simple sequence repeat, random amplified polymorphic DNA, single-nucleotide polymorphism, restricted fragment length polymorphisms, sequence-related amplified polymorphism, single-strand conformational polymorphism and sequence characterized amplified region [100][112]. These different molecular identifications used in different taxonomic classifications of Ganoderma have caused great improvements and provide information for the further research of Ganoderma [18][90][101][18,102,113]. Ganoderma has long been treated as one of the most important medicinal fungi worldwide [39], and laccate species of Ganoderma have been considered for centuries [102][114], i.e., G. lucidum complex have been used as medicinal mushrooms in traditional Chinese medicine [103][115]. Anyhow, the species concept of the G. lucidum complex lacks agreement in morphology, and the taxonomy of this species complex is thus problematic, and this ultimately limits both further research on this complex and their medicinal usefulness. For example, the widely used medicinal species in biochemical and pharmaceutical studies have been assumed to be G. lucidum, but evidence has emerged that this medicinal species is, in fact, a different species [104][116] and was described as G. lingzhi [94][106]. The taxonomic position of the G. lucidum complex has long been subjected to debate [9][18][90][101][105][9,18,102,113,117] and different opinions have been expressed regarding the members and their validity in the complex. Haddow [106][118] treated G. sessile as a synonym of G. resinaceum, while Overholts [107][119] pointed out that G. lucidum should be the correct name of the specimens classified as G. sessile. Ganoderma lucidum has also been considered as the correct name over its later synonym G. tsugae [106][108][118,120]. However, with the support of mating tests, Nobles [109][121] pointed out that the specimens classified as G. lucidum in the USA represented G. sessile. All names of the G. lucidum complex in East Africa were parsimoniously treated as the ‘‘G. lucidum group’’, because of the lack of morpho-taxonomic solution to the problem in this complex [7]. Asian specimens classified as G. lucidum were divided into two clades; both were separated from European G. lucidum [110][122], with one clade being composed of tropical collections represented by G. multipileum, while the other clade was unknown [110][122], yet later recognized as G. sichuanense [111][123]. It was found that the holotype of G. sichuanense was not conspecific with the unknown clade, and the unknown clade was identified as a new species, G. lingzhi, which also is the most widely cultivated species in China [94][106]. Meanwhile, the distribution of genuine G. lucidum in China was also confirmed [94][112][106,124]. The taxonomy of the G. lucidum complex remains problematic even after several decades of debates [99][111]. Most of the studies previously conducted were focused on the species in a continent or specific region [94][111][106,123], or the phylogeny was described with low resolution to certain clades [104][112][116,124]. On the other hand, a strong phylogeny together with species originally described as from the USA is greatly needed, as most of these species are old and never referred to in any phylogenetic analyses.2. Triterpenoids
2.1. Biosynthesis of Triterpenoids
Triterpenoids belong to a large and structurally diverse class of natural products [113][125]. Basidiomycetes are considered a main source for triterpenoids. Compared to plants, very few types of triterpenoid skeletons have been reported in basidiomycetes, thus more research is needed [114][126]. Triterpenoids extracted from Ganoderma spp. are named as Ganoderma triterpenoids (GTs) [113][125]. Ganoderma triterpenoids (GTs) isolated from the fruiting bodies, cultured mycelia and basidiospores of Ganoderma [115][116][117][127,128,129] belong to the lanostane-type triterpenoids and are one of the major chemical constituents in Ganoderma. Studies have confirmed that GTs are biosynthesized using isoprenoid pathways [118][119][130,131]. This pathway was considered to start from acetyl-coenzyme A and termed as a Mevalonate pathway (MVA) [120][132]. The MVA pathway (Figure 1) is one of the important metabolic pathways that can be divided into four main processes: conversion, construction, condensation, and postmodification [35]. The initial step is the transformation of acetyl-coenzyme A to isopentenyl pyrophosphate (IPP). Then the activities of different prenyltransferases produce farnesyl pyrophosphate (FPP), geranyl pyrophosphate (GPP) and geranylgeranyl pyrophosphate (GGPP), which are higher-order terpenoid building blocks from this IPP precursor. Then, these junction mediators can self-condense, and are also used in alkylation processes to produce prenyl side chains (for a range of nonterpenoids) or create a ring (to build the basic skeletons of triterpenoids). Ultimately, oxidation and reduction reactions, conjugation, isomerization or other secondary reactions magnify the distinctive characteristics of the triterpenoids [35][121][122][35,133,134].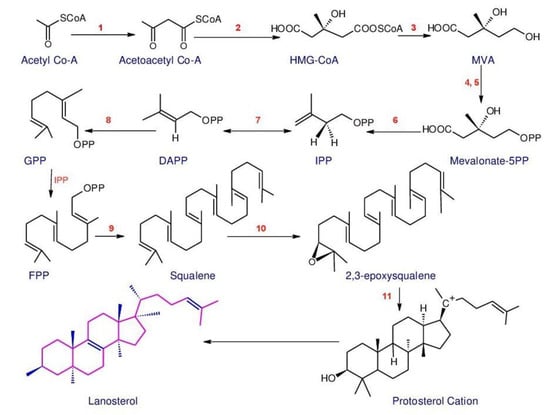
Figure 1. Outline of the MVA and lanostane-type triterpenoids biosynthesis. Enzymes involved in the pathway are: 1. Acetyl-CoA acetyltransferase, AACT; 2. 3-Hydroxy-3-methylglutaryl-CoA synthase, HMGS; 3. 3-Hydroxy-3-methylglutaryl-CoA reductase, HMGR; 4. Mevalonate kinase, MK; 5. Phosphomevalonate kinase, MPK; 6. Phosphomevalonate decarboxylase, MVD; 7. Isopentenyldiphosphate isomerase, IDI; 8. Farnesyl diphosphate synthase, FPPs; 9. Squalene synthase, SQS; 10. Squalene monooxygenase, SE; 11. 2,3-Oxidosqualene-lanosterol cyclase, OSC (The structures were redrawn in ACD/ChemSketch: Freeware: 2012).
2.2. Structures and Bioactivities of Triterpenoids
Triterpenes are a major class of widely dispersed secondary metabolites in nature [132][144]. Triterpenoids structures with a carbon skeleton are considered to be derived from the acyclic precursor squalene [133][145]. More than 30,000 structures of triterpenes [134][146] such as dammarane, lanostane, lupine, oleanane and ursane types have been isolated and identified [135][147]. The structures of triterpenoids isolated from Ganoderma spp. are complicated. These compounds consist with lanostane carbon skeleton and pentacyclic triterpenoids (Figure 2). According to the number of carbon atoms in their skeleton, GTs can be divided into three types viz. C30, C27 and C24 [136][148]. On the basis of the substituent groups, they are classified into different groups such as triterpenoid acids, triterpenoid alcohols and triterpenoid lactones [136][148].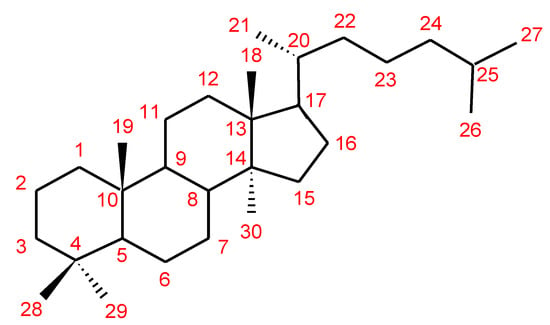
Figure 2. Chemical structure of lanostane triterpene.
2.2.1. C30 Triterpenoids
The majority of GTs was C30 triterpenoids on the basis of the biosynthetic pathway of GTs. Because of the oxidation and reduction processes, their structures should be divided into six groups: 8,9-ene, 8,9-dihydro, 8,9-epoxy, 7(8),9(11)-diene, triterpenoid saponins and rearranged novel skeleton triterpenoids.8,9-ene-triterpenoids
This type of GT is the lanostane-type triterpenoid with a double bond between C-8 and C-9. In addition, C-3, C-7, C-11 and C-15 were generally substituted by the hydroxyl or carbonyl groups. The minority of compounds possessed hydroxyl or carbonyl groups at C-12. Different oxidation and reduction can happen in the side chain. Except the above structural features, compounds ganodermacetal (44) and methyl ganoderate A acetonide (45) possessed an uncommon acetonide unit. Compound 44 was isolated from the basidiomycete G. amboinense, and was a natural product, but not an artefact, which resulted from the acetalization of native ganoderic acid C (3) and with acetone not being used during the isolation procedure [141][153]. However, compound 45 isolated from the fruiting bodies of G. lucidum was reported to be most likely not of natural origin due to the utilization of acetone during the isolation procedure [142][154]. Several studies on the bioactivity of triterpenoids showed that ganoderic acids had significant biological activities [95][143][144][145][146][147][107,155,156,157,158,159] (Table 1). Ganoderic acid DM (50) displayed stronger 5α-reductase inhibitory activity (IC50 value of 10.6 μM) than the positive control (α-linolenic acid, 116 μM). Meanwhile, compared to its methyl derivative, the inhibitory activities of 5α-reductase at 20 μM were 55% and 3% for 50 and its derivative, suggesting that the carboxyl group of the side chain for 50 is essential to elicit the inhibitory activity [143][155]. Liu et al. [144][156] evaluated the structure –activity relationship for inhibition of 5α-reductase using ganoderic acid A (1), B (2), C (3), D (19), I (5) and DM (50). The results showed that the presence of the carbonyl group at C-3, and of the α,β-unsaturated carbonyl group at C-26, was characteristic of almost all inhibitors.Table 1. 8,9-double-bond triterpenoids and bioactivities from Ganoderma.
| No. | Trivial Names | Bioactivities (IC50/MIC or ED50) | Sources Ganoderma Species |
References |
|---|---|---|---|---|
| 1. | Ganoderic acid A | Promising anticancer agent (via potent inhibitory effect on JAK/STAT3 pathway) | G. lucidum, G. tsugae |
[148][149][150][165,166,167] |
| 2. | Ganoderic acid B | Moderately active inhibitor against HIV-1 PR (0.17 mM) | G. lucidum, G. tsugae |
[148][149][165[151],166,168] |
| 3. | Ganoderic acid C | Suppressed LPS-induced TNF-α (IC50 = 24.5 µg/mL) production through down-regulating MAPK, NF-kappa B and AP-1 signaling pathways in macrophages | G. lucidum, G. tsugae |
[148][152][165,169] |
| 4. | Ganoderic acid G | Antinociceptive effect | G. lucidum | [153][154][170,171] |
| 5. | Ganoderic acid I | Cytotoxicity against Hep G2 cells (IC50 = 0.26 mg/mL), HeLa cells (IC50 = 0.33 mg/mL), Caco-2 cells (IC50 = 0.39 mg/mL) |
G. lucidum | [153][155][170,172] |
| 6. | Ganolucidic acid A | - | G. lucidum | [156][173] |
| 7. | Ganolucidic acid B | - | G. lucidum | [157][174] |
| 8. | Methyl ganoderate M | - | G. lucidum | [158][175] |
| 9. | Methyl ganoderate N | - | G. lucidum | [158][175] |
| 10. | Methyl ganoderate O | - | G. lucidum | [158][175] |
| 11. | Methyl ganoderate K | - | G. lucidum | [158][175] |
| 12. | Compound B9 | - | G. lucidum | [158][175] |
| 13. | Methyl ganoderate H | - | G. lucidum | [158][175] |
| 14. | Ganoderic acid α | Anti-HIV protease (0.19 mM) | G. lucidum | [151][168] |
| 15. | 3-O-acetylganoderic acid B | - | G. lucidum | [159][176] |
| 16. | Ethyl 3-O-acetylganoderate B | - | G. lucidum | [159][176] |
| 17. | 3-O-Acetylganoderic acid K | - | G. lucidum | [159][176] |
| 18. | Ethyl ganoderate J | - | G. lucidum | [159][176] |
| 19. | Ganoderic acid D | Cytotoxicity against HeLa cells (17.3 μM), 5α-reductase inhibition—NE | G. lucidum, G. applanatum, G. tsugae |
[144][148][156,165, [160]177[161][162],178,179] |
| 20. | Ganoderic acid F | Cytotoxicity against HeLa cells (19.5 μM) | G. lucidum | [160][162][177,179] |
| 21. | Ganoderic acid E | Cytotoxicity against tumor cell lines [Hep G2 (1.44 × 10−4 μM), HepG2,2,15 (1.05 × 10−4 μM), κB—NE, CCM2 (31.25 μM), p388 (5.02 μΜ)] | G. lucidum, G. tsugae |
[148][160][165,177, [163]180] |
| 22. | Ganoderic acid Df | Human aldose reductase inhibitory activity (22.8 μM/mL) | G. lucidum | [164][162] |
| 23. | Ganosporeric acid A | - | G. lucidum | [165][181] |
| 24. | Ganohainanic acid A | Cytotoxicity—NE | G. hainanense | [62] |
| 25. | Acetyl ganohainanic acid A | Cytotoxicity—NE | G. hainanense | [62] |
| 26. | Ganohainanic acid B | Cytotoxicity—NE | G. hainanense | [62] |
| 27. | Ganohainanic acid C | Cytotoxicity—NE | G. hainanense | [62] |
| 28. | Ganohainanic acid D | Cytotoxicity—NE | G. hainanense | [62] |
| 29. | Acetyl ganohainanic acid D | Cytotoxicity—NE | G. hainanense | [62] |
| 30. | Methyl ganoderate D | - | G. lucidum | [166][167][182,183] |
| 31. | Methyl ganoderate E | - | G. lucidum | [168][184] |
| 32. | Methyl ganoderate F | Inhibitory effects on EBV-EA induction (289 mol ratio/32 pmol TPA) | G. lucidum | [168][169][184,185] |
| 33. | 12β-Acetoxy-3β,7β-dihydroxy-11,15,23-trioxolanost-8-en-26-oic acid butyl ester | Antimicrobial [Staphylococcus aureus ATCC 6538 (68.5 μM) and Bacillus subtilis ATCC6633 (123.8 μM)] |
G. lucidum | [170][186] |
| 34. | 12β-acetoxy-3,7,11,15,23-pentaoxolanost-8-en-26-oic acid butyl ester | Antimicrobial—NE | G. lucidum | [170][186] |
| 35. | n-Butyl ganoderate H | Selective cholinesterase inhibition | G. lucidum | [142][154] |
| 36. | Butyl ganoderate A | Cytotoxicity against 3T3-L1 cells —NE | G. lucidum | [167][183] |
| 37. | Butyl ganoderate B | Cytotoxicity against 3T3-L1 cells —NE | G. lucidum | [167][183] |
| 38. | 3β,7β,15β-Trihydroxy-11,23-dioxo-lanost- 8,16-dien-26-oic acid |
Anti-AChE—NE | G. tropicum | [171][187] |
| 39. | 3β,7β,15β-Trihydroxy- 11,23-dioxo-lanost-8,16- dien-26-oic acid methyl ester |
Anti-AChE (15.72%) | G. tropicum | [171][187] |
| 40. | 3β,15β-Dihydroxy- 7,11,23-trioxo-lanost- 8,16-dien-26-oic acid methyl ester |
Anti-AChE—NE | G. tropicum | [171][187] |
| 41. | Ganoderenic acid G | - | G. applanatum | [172][188] |
| 42. | Ganoderenic acid F | - | G. applanatum | [172][188] |
| 43. | Methyl ganoderate I | - | G. applanatum | [172][188] |
| 44. | Ganodermacetal | Toxic activity against brine shrimp larvae | G. amboinense | [141][153] |
| 45. | Methyl ganoderate A acetonide | Anti-AChE (18.35 μM), anti-BChE—NE | G. lucidum | [142][154] |
| 46. | Ganoderic acid Z | Cytotoxicity | G. lucidum | [173][189] |
| 47. | Ganoderic acid W | Cytotoxicity | G. lucidum | [173][189] |
| 48. | Ganoderic acid V | Cytotoxicity | G. lucidum | [173][189] |
| 49. | Ganoderic acid U | - | G. lucidum | [174][190] |
| 50. | Ganoderic acid DM | 5α-Reductase inhibition (10.6 μM), anti-androgen and anti-proliferative activities, osteoclastogenesis inhibitor, inhibits prostate cancer cell growth, inhibits breast cancer cell growth |
G. lucidum, G. sinense |
[95][145][107,157, [146]158[175],160, [176]191[177],192] |
| 51. | 7-Oxo-ganoderic acid Z2 | - | G. resinaceum | [178][193] |
| 52. | 7-Oxo-ganoderic acid Z3 | - | G. resinaceum | [178][193] |
| 53. | Ganoderic acid GS-1 | Anti-HIV protease (58 µM) | G. sinense | [177][192] |
| 54. | Ganoderic acid GS-2 | Anti-HIV protease (30 μM) | G. sinense | [177][192] |
| 55. | Ganoderic acid GS-3 | Anti-HIV protease—NE | G. sinense | [177][192] |
| 56. | Ganoderic acid Ma | - | G. lucidum | [179][194] |
| 57. | Ganoderic acid Mb | - | G. lucidum | [179][194] |
| 58. | Ganoderic acid Mc | - | G. lucidum | [179][194] |
| 59. | Ganoderic acid Md | - | G. lucidum | [179][194] |
| 60. | Ganoderic acid Mg | - | G. lucidum | [174][190] |
| 61. | Ganoderic acid Mh | - | G. lucidum | [174][190] |
| 62. | Ganoderic acid Mi | - | G. lucidum | [174][190] |
| 63. | Ganoderic acid Mj | - | G. lucidum | [174][190] |
| 64. | 3α,22β-Diacetoxy-7α-hydroxyl-5α-lanost-8,24E-dien-26-oic acid | Cytotoxicity against 95D (IC50 = 23 μM/mL), HeLa human tumor cell lines (IC50 = 14.7 μM/mL) |
G. lucidum (mycelia) | [180][195] |
| 65. | 7-O-Ethyl ganoderic acid O | Cytotoxicity against 95D (46.7 μM), HeLa cells (59.1 μM) | G. lucidum | [181][196] |
| 66. | Ganorbiformin B | - | G. orbiforme | [36] |
| 67. | Ganorbiformin C | - | G. orbiforme | [36] |
| 68. | Ganorbiformin D | Cytotoxicity (against NCIH187, MCF-7, and κB—NE), nonmalignant Vero cells, antimalarial, antitubercular—NE | G. orbiforme | [36] |
| 69. | Ganorbiformin E | Cytotoxicity against NCIH187 (70 µM), MCF-7, κB—NE, nonmalignant Vero cells, antimalarial, antitubercular—NE | G. orbiforme | [36] |
| 70. | Ganorbiformin F | Cytotoxicity against NCIH187 (44 µM), MCF-7—NE and κB (63 µM), nonmalignant Vero cells (36 µM), antimalarial, antitubercular—NE | G. orbiforme | [36] |
| 71. | 7β,23ξ-Dihydroxy-3,11,15-trioxolanosta-8,20E(22)-dien-26-oic acid | - | G. applanatum | [38] |
| 72. | Methyl ganoderenate D | - | G. applanatum | [38] |
| 73. | 3β,7β,20,23ξ-Tetrahydroxy-11,15- dioxolanosta-8-en-26-oic acid |
- | G. applanatum | [38] |
| 74. | 7β,20,23ξ-Trihydroxy-3,11,15- trioxolanosta-8-en-26-oic acid |
- | G. applanatum | [38] |
| 75. | Ganoderic acid L | - | G. lucidum | [182][197] |
| 76. | Methyl ganoderate L | - | G. lucidum | [182][197] |
| 77. | Ganolucidic acid γa | PXR-mediated CYP3A4 expression—NE | G. sinense | [183][198] |
| 78. | Ganolucidate F | PXR-mediated CYP3A4 expression | G. sinense | [183][198] |
| 79. | Ganolucidic acid D | Cytotoxicity on tumor growth cells—NE | G. lucidum | [182][184][197,199] |
| 80. | Methyl ganolucidate D | - | G. lucidum | [182][197] |
| 81. | Hainanic acid A | Cytotoxicity—NE | G. hainanense | [62] |
| 82. | Hainanic acid B | Cytotoxicity—NE | G. hainanense | [62] |
| 83. | Ganoderic acid γ | Cytotoxicity against tumor cell growth Meth-A (ED50 = 15.6 μg/mL), LLC—NE | G. lucidum | [184][199] |
| 84. | Ganoderic acid δ | Cytotoxicity against tumor cell growth Meth-A and LLC—NE | G. lucidum | [184][199] |
| 85. | Ganoderic acid ε | Cytotoxicity against tumor cell growth Meth-A (ED50 = 12.2 μg/mL), LLC—NE | G. lucidum | [184][199] |
| 86. | Ganoderic acid ζ | Cytotoxicity against tumor cell growth Meth-A and LLC—NE | G. lucidum | [184][199] |
| 87. | Ganoderic acid η | Cytotoxicity against tumor cell growth Meth-A and LLC—NE | G. lucidum | [184][199] |
| 88. | Ganoderic acid θ | Cytotoxicity against tumor cell growth Meth-A (ED50 = 5.7 μg/mL), LLC (ED50 = 15.2 μg/mL) | G. lucidum | [184][199] |
| 89. | Ganoderiol G | - | G. lucidum | [185][200] |
| 90. | Ganoderiol H | - | G. lucidum | [185][200] |
| 91. | Ganoderiol I | - | G. lucidum | [185][200] |
| 92. | 24S,25R-Dihydroxy-3,7-dioxo-8-en-5α-lanost-26-ol | Cytotoxicity—NE | G. hainanense | [62] |
| 93. | Ganoderone A | Antiviral: influenza A—NE, HSV (0.3 μg/mL) | G. pfeifferi | [46] |
| 94. | Ganoderone C | Antiviral—NE | G. pfeifferi | [46] |
| 95. | 3,7,24-Trioxo-5α-lanost- 8,25-dien-26-ol |
Cytotoxicity against HL-60 (15.70 µM), SMMC-7721 (15.52 µM), A-549 (15.81 µM), MCF-7 (20.08 µM), SW480—NE | G. hainanense | [62] |
| 96. | Hainanaldehyde A | Cytotoxicity—NE | G. hainanense | [62] |
| 97. | 21-Hydroxy-3,7-dioxo-5α-lanost-8,24E-dien-26-ol | Cytotoxicity—NE | G. hainanense | [62] |
| 98. | 3β,11α-Dihydroxy-7-oxo-5α-lanost-8,24E-dien-26-ol | Cytotoxicity—NE | G. hainanense | [62] |
| 99. | Lucialdehyde D | - | G. lucidum, G. pfeifferi |
[46][186][46,201, [187]202] |
| 100. | Ganoderiol J | - | G. sinense | [183][198] |
| 101. | 16α,26-Dihydroxylanosta-8,24-dien-3-one | Cytotoxicity against K-562 cells (13.3 μg/mL) | G. hainanense | [75] |
| 102. | Lucidadiol | Antiviral: influenza virus type A (ED50 = 0.22 mmol/L), HSV—NE |
G. lucidum, G. pfeifferi |
[188][189][203,204] |
| 103. | Sinensoic acid | - | G. sinense | [190][205] |
| 104. | Tsugaric acid C | Cytotoxicity—NE | G. tsugae | [191][206] |
| 105. | Colossolactone A | Moderate cytotoxicity against L-929, K-562, HeLa cells—NE, anti-inflammatory properties | G. colossum | [192][207] |
| 106. | Ganoderenicfy A | Promoting angiogenesis activities | G. applanatum | [193][208] |
| 107. | Colossolactone I | Moderate cytotoxicity against HCT-116 colorectal cancer cells, Antimalarial: Plasmodium falciparum—NE | G. colossum | [194][195][92,209, [196]210] |
| 108. | Colossolactone II | Low cytotoxicity against HCT-116 colorectal cancer cells | G. colossum | [194][195][92,209] |
| 109. | Ganodermalactone E | Antimalarial: Plasmodium falciparum—NE | G. colossum | [196][210] |
| 110. | Colossolactone B | Moderate cytotoxicity against L-929, K-562, and HeLa cells, antimicrobial—NE, antibacterial—NE | G. colossum | [192][196][207,210, [197]211] |
| 111. | Methyl ganolucidate A | - | G. lucidum | [153][157][170,174] |
| 112. | Methyl ganolucidate B | - | G. lucidum | [153][157][170,174] |
| 113. | Methyl ganoderate A | - |
G. lucidum | [166][182] |
| 114. | Methyl ganoderate B | - | G. lucidum | [166][182] |
| 115. | Methyl ganoderate C | - | G. lucidum | [166][182] |
| 116. | Methyl ganoderate C2 | - | G. lucidum (dried fruit bodies) |
[198][212] |
| 117. | Compound B8 | - | G. lucidum (dried fruit bodies) |
[198][212] |
| 118. | 3β-Oxo-formyl-7β,12β-dihydroxy-5α-lanost-11,15,23-trioxo-8-en(E)-26-oic acid | - | G. lucidum (fruit bodies) |
[199][213] |
| 119. | Ganoderic acid B8 | Cytotoxicity against LLC—NE, T47-D—NE, S-180—NE, Meth-A—NE | G. lucidum (fruit bodies) |
[200][214] |
| 120. | Ganoderic acid C1 | Inhibitory activity against HIV-PR (0.18 mM) | G. lucidum (fruit bodies) |
[151][168[200],214] |
| 121. | 12β-Acetoxy-3,7,11,15,23-pentaoxo-5α-lanosta-8-en-26-oic acid ethyl ester | Cytotoxicity against human HeLa cervical cancer cell lines (63 μM) | G. lucidum | [201][215] |
| 122. | 3β,7β-Dihydroxy-12β-acetoxy-11,15,23-trioxo-5α-lanosta-8-en-26-oic acid methyl ester | - | G. lucidum | [32] |
| 123. | 3β-Hydroxy-7,11,12,15,23-pentaoxolanost-8-en-26-oic acid | Cytotoxic against p388 cell (9.85 μM), HeLa cell (17.10 μM), BEL-7402 cell (51.00 μM), SGC-7901 cells (42.00 μM) |
G. lucidum (fruit bodies) |
[202][216] |
| 124. | Ganoderic acid H | Inhibitory activity against HIV-PR (0.20 mM) | G. lucidum (fruit bodies) |
[160][203][177,217] |
| 125. | Ganoderic acid K | Cytotoxicity against p388 cell (13. 8 μM), HeLa cell (8.23 μM); BEL-7402 cell (16.5 μM), SGC-7901cell (21.0 μM) |
G. lucidum (fruit bodies) |
[204][218] |
| 126. | Ganoderic acid AM1 | Cytotoxicity against p388 cell (13. 2 μM), HeLa cell (9.75 μM), BEL-7402 cell (20.9 μM), SGC-7901 cell (23.0 μM) |
G. lucidum (fruit bodies) |
[204][218] |
| 127. | Ganoderic acid J | Cytotoxicity against p388 cell (15. 8 μM), HeLa cell (12.2 μM), BEL-7402 cell (25.2 μM), SGC-7901 cell (20.2 μM) |
G. lucidum (fruit bodies) |
[204][218] |
| 128. | Ganoderic acid AP2 | - | G. applanatum (fruit bodies) |
[161][178] |
| 129. | 23S-Hydroxy-3,7,11,15-tetraoxolanost-8,24E-diene-26-oic acid | Cytotoxicity against p388 cell (15.7 μM), HeLa cell (9.72 μM), BEL-7402 cell (25.6 μM), SGC-7901 cell (23.1 μM) | G. lucidum (fruit bodies) |
[204][218] |
| 130. | 7-Oxoganoderic acid Z | Inhibitory activities against the HMG-CoA reductase (22.3 μM), acyl CoA acyltransferase (5.5 μM) |
G. lucidum (fruit bodies) |
[205][219] |
| 131. | Ganoderic acid LM2 | Potent enhancement of ConA-induced mice splenocytes proliferation in vitro | G. lucidum (fruit bodies) |
[206][220] |
| 132. | Lucialdehyde B | Cytotoxic effect on tested tumor cells | G. lucidum (fruit bodies) |
[200][214] |
| 133. | Lucialdehyde C | Cytotoxicity against LLC, T-47D (10.7 µg/mL), Sarcoma 180 (4.7 µg/mL), Meth-A tumor cells (3.8 µg/mL) |
G. lucidum (fruit bodies) |
[200][214] |
| 134. | Ganoderic acid β | HIV-I protease inhibitory activity (20 µM) | G. lucidum (spores) | [207][221] |
| 135. | Ganolucidic acid E | - | G. lucidum (fruit bodies) |
[185][200] |
| 136. | Ganoderal B | - | G. lucidum | [208][222] |
| 137. | 11α-Hydroxy-3,7-dioxo-5α-lanosta-8,24(E)-dien-26-oic acid | Cytotoxicity against human HeLa cervical cancer cell lines (123 μM) | G. lucidum | [201][215] |
| 138. | 11β-Hydroxy-3,7-dioxo-5α-lanosta-8,24(E)-dien-26-oic acid | Cytotoxicity against human HeLa cervical cancer cell lines (51 μM) | G. lucidum | [201][215] |
| 139. | Lucidal | - | G. lucidum (cultured fruit bodies) |
[188][203] |
| 140. | Lucialdehyde E | Cytotoxic activity against esophageal tumor EC109 cell line (18.7 mg/mL) | G. lucidum (spores) | [187][202] |
| 141. | 3α,22β-Diacetoxy-7α-hydroxyl-5α-lanost-8,24E-dien-26-oic acid | Cytotoxicity against HeLa cell lines (14.7 μM), 95D cell lines (23.01 μM) | G. lucidum (mycelial mat) |
[180][195] |
| 142. | Ganoderic acid O | - | G. lucidum (cultured mycelium) |
[209][223] |
| 143. | 7-O-Methylganoderic acid O | - | G. lucidum (cultured mycelium) |
[209][223] |
| 12β-Acetoxy-3β-hydroxy-7,11,15,23- tetraoxo-lanost-8,20E-diene-26-oic acid |
Cytotoxicity against human cancer cell p388 (12.7 µM), HeLa cell (8.72 µM), BEL-7402 (24.2 µM), SGC-7901 (18.7 µM) | G. lucidum (fruit bodies) |
[204][218] | |
| 145. | 23-Dihydroganoderenic acid D | - | G. applanatum (fruit bodies) |
[38] |
| 146. | Ganoderenic acid A | - | G. lucidum (dried fruit bodies) |
[160][177] |
| 147. | Ganoderenic acid B | - | G. lucidum (dried fruit bodies) |
[160][177] |
| 148. | Ganoderenic acid C | - | G. lucidum (dried fruit bodies) |
[160][177] |
| 149. | Ganoderenic acid D | - | G. lucidum (dried fruit bodies) |
[160][177] |
| 150. | 12β-Acetoxy-7β-hydroxy-3,11,15,23- tetraoxo-5α-lanosta-8,20-dien-26-oic acid |
Cytotoxicity against human HeLa cervical cancer cell lines—NE | G. lucidum | [201][215] |
| 151. | Methy ganoderenate H | - | G. applanatum (fruit bodies) |
[172][188] |
| 152. | Methyl ganoderenate I | - | G. applanatum (fruit bodies) |
[172][188] |
| 153. | 12β-Acetoxy-3β,7β-dihydroxy-11,15,23-trioxo-5α-lanosta-8,20-dien-26-oic acid | - | G. lucidum | [32] |
| 154. | Methyl ganoderate G | - | G. lucidum | [153][170] |
| 155. | Compound C5 | - | G. lucidum (fruit bodies) |
[203][217] |
| 156. | Compound C6 | - | G. lucidum (fruit bodies) |
[203][217] |
| 157. | Ganoderic acid AP3 | - | G. applanatum (fruit bodies) |
[161][178] |
| 158. | 23-Dihydroganoderic acid I | - | G. applanatum (fruit bodies) |
[38] |
| 159. | 23-Dihydroganoderic acid N | - | G. applanatum (fruit bodies) |
[38] |
| 160. | 20-Hydroxylganoderic acid G | - | G. lucidum (fruit bodies) |
[210][224] |
| 161. | Lucidumol A | HIV-I protease inhibitory activity—NE | G. lucidum (spores) | [207][221] |
| 162. | Ganoderiol C | - | G. lucidum (fruit bodies) |
[185][200] |
| 163. | Ganoderiol D | - | G. lucidum (fruit bodies) |
[185][200] |
| 164. | Ganoderitriol M | - | G. lucidum (fruit bodies) |
[211][225] |
| 165. | Tsugaric acid A | - | G. tsugae | [212][226] |
| 166. | Tsugarioside A | Cytotoxicity against PLC/PRF/5 (ED50 = 6.5 μg/mL), T-24 (ED50 = 8.6 μg/mL), HT-3 (ED50 = 7.2 μg/mL), SiHa (ED50 = 9.5 μg/mL) | G. tsugae (fruit bodies) |
[191][206] |
| 167. | 3-Oxo-5α-lanosta-8,24-dien-21-oic acid | Cytotoxicity—NE | G. resinaceum (fruit bodies) |
[213][227] |
| 168. | 3β-Hydroxy-5α-lanosta-8,24-dien-21-oic acid | Cytotoxicity against T-24 (ED50 = 4.4 μg/mL), HT-3 (ED50 = 3.5 μg/mL), SiHa (ED50 = 5.5 μg/mL), CaSKi (ED50 = 6.2 μg/mL) | G. tsugae (fruit bodies) |
[191][206] |
| 169. | 3β,7β-Dihydroxy-11,15,23-trioxolanost-8,16-dien-26-oic acid | - | G. lucidum (fruit bodies) |
[188][203] |
| 170. | 3β,7β-Dihydroxy-11,15,23-trioxolanost-8,16-dien-26-oic acid methyl ester | - | G. lucidum (fruit bodies) |
[188][203] |
| 171. | 12β-Acetoxy-3β,7β-dihydroxy-11,15,23-trioxolanost-8,16-dien-26-oic acid | - | G. lucidum (fruit bodies) |
[214][228] |
| 172. | Methyl ganoderate AP | - | G. applanatum (fruit bodies) |
[172][188] |
| 173. | Ganoderiol E | - | G. lucidum (fruit bodies) |
[185][200] |
| 174. | Epoxyganoderiol A | - | G. lucidum | [208][222] |
| 175. | 3α-Carboxyacetoxy-24-methylene-23-oxolanost-8-en-26-oic acid | Cytotoxicity—NE | G. applanatum (fruit bodies) |
[215][229] |
| 176. | 3α-Carboxyacetoxy-24-methyl-23- oxolanost-8-en-26-oic acid |
Cytotoxicity—NE | G. applanatum (fruit bodies) |
[215][229] |
| 177. | 3-Epipachymic acid | - | G. resinaceum (fruit bodies) |
[213][227] |
| 178. | 3β,15α-Diacetoxylanosta-8,24-dien-26-oic acid | - | G. lucidum (mycelia) | [216][230] |
| 179. | Ganoderic acid V1 | - | G. lucidum | [217][231] |
| 180. | Tsugaric acid B | - | G. tsugae | [212][226] |
| 181. | Methyl ganoderenate E | - | G. lucidum (fruit bodies) |
[158][175] |
| 182. | Lucidumol D | Selective anti-proliferative and cytotoxic effects | G. lingzhi | [218][232] |
| 183. | Lucidumol C | Selective anti-proliferative and cytotoxic effects | G. lingzhi | [218][232] |
| 184. | Leucocontextin A | - | G. leucocontextum | [219][233] |
| 185. | Leucocontextin B | - | G. leucocontextum | [219][233] |
| 186. | Leucocontextin C | - | G. leucocontextum | [219][233] |
| 187. | Leucocontextin D | - | G. leucocontextum | [219][233] |
| 188. | Leucocontextin E | - | G. leucocontextum | [219][233] |
| 189. | Leucocontextin F | - | G. leucocontextum | [219][233] |
| 190. | Leucocontextin G | - | G. leucocontextum | [219][233] |
| 191. | Leucocontextin H | - | G. leucocontextum | [219][233] |
| 192. | Leucocontextin I | - | G. leucocontextum | [219][233] |
| 193. | Leucocontextin R | Cytotoxicity against K562 and MCF-7 cell lines (IC50 = 20–30 μM) | G. leucocontextum | [219][233] |
| 194. | Ganoleuconin A | Cytotoxicity against K562 (17.8 μM), PC-3 cell lines—NE | G. leucocontextum | [34] |
| 195. | Ganoleuconin B | Cytotoxicity against K562 (19.7 μM), PC-3 cell lines—NE | G. leucocontextum | [34] |
| 196. | Ganoleuconin E | Cytotoxicity against K562 and PC-3 cell lines—NE | G. leucocontextum | [34] |
| 197. | Ganoleuconin G | Cytotoxicity against K562 (11.4 μM), PC-3 cell lines (132.4 μM) | G. leucocontextum | [34] |
| 198. | Ganoleuconin H | Cytotoxicity against K562 (115.4 μM), PC-3 cell lines (24.2 μM) | G. leucocontextum | [34] |
| 199. | Ganoleuconin I | Cytotoxicity against K562 and PC-3 cell lines—NE | G. leucocontextum | [34] |
| 200. | Ganoderenicfy B | Promoting angiogenesis activities | G. applanatum | [193][208] |
| 201. | (24E)-15α,26-Dihydroxy-3-oxo-lanosta-8,24-diene | Antimycobacteria (50 μg/mL), cytotoxicity (5.9 μg/mL) | G. casuarinicola | [220][234] |
| 202. | (24E)-7α,26-Dihydroxy-3-oxo-lanosta-8,24-diene. | Antimalarial activity (IC50 = 9.7 μg/mL) | G. casuarinicola | [220][234] |
| 203. | (24E)-3-Oxo-7α,15α,26-trihy-droxylanosta-8,24-diene | Antimalarial activity (IC50 = 9.2 μg/mL) | G. casuarinicola | [220][234] |
| 204. | (24E)-3β-Acetoxy-15α,26-dihydroxylanosta-8,24-diene | Antimycobacteria (25 μg/mL), cytotoxicity (6 μg/mL) | G. casuarinicola | [220][234] |
| 205. | (24E)-3β-Acetoxy-7α,15α,26- trihydroxylanosta-8,24-diene |
Antimycobacteria (25 μg/mL), cytotoxicity (9 μg/mL) | G. casuarinicola | [220][234] |
| 206. | (24E)-3β,7α,15α,26-Tetra-hydroxylanosta-8,24-diene | - | G. casuarinicola | [220][234] |
| 207. | 7β,15α,20-Trihydroxy-3,11,23-trioxo-5α-lanosta-8-en-26-oic acid | - | G. lucidum | [221][235] |
| 208. | Ganoderic acid XL3 | - | G. theaecolum | [222][236] |
| 209. | Ganoderic acid XL4 | - | G. theaecolum | [222][236] |
| 210. | Ganodecalone A | Cytotoxicity against K562 (17.22 µM) | G. calidophilum | [53] |
| 211. | Ganoderic acid C6 | - | G. lucidum (fruit bodies) |
[65] |
| 212. | Ganoderic acid D1 | - | G. lucidum (fruit bodies) |
[65] |
| 213. | Ganoderic acid M | - | G. lucidum (fruit bodies) |
[65] |
| 214. | Ganoderic acid N | - | G. lucidum (fruit bodies) |
[65] |
| 215. | 12-Hydroxylganoderic acid C2 | - | G. lucidum (fruit bodies) |
[65] |
| 216. | 3-Acetylganoderic acid H | - | G. lucidum (fruit bodies) |
[65] |
| 217. | 12-Acetoxyganoderic acid D | - | G. lucidum (fruit bodies) |
[65] |
| 218. | 12-Hydroxyganoderic acid D | - | G. lucidum (fruit bodies) |
[65] |
| 219. | 12-Acetoxyganoderic acid F | - | G. lucidum (fruit bodies) |
[65] |
| 220. | 12β-Hydroxy-3,7,11,15,23-pentaoxo-5α-lanosta-8-en-26-oic acid | - | G. lucidum (fruit bodies) |
[65] |
| 221. | 12-Hydroxy-3,7,11,15,23-pentaoxo-lanost-8-en-26-oic acid | - | G. lucidum (fruit bodies) |
[65] |
| 222. | 12,15-Bis(acetyloxy)-3-hydroxy-7,11,23-trioxo-lanost-8-en-26-oic acid | - | G. lucidum (fruit bodies) |
[65] |
| 223. | Methyl ganoderate J | - | G. lucidum (fruit bodies) |
[65] |
| 224. | Methyl-O-acetylganoderate C | - | G. lucidum (fruit bodies) |
[65] |
| 225. | Methyl ganoderate C1 | - | G. lucidum (fruit bodies) |
[65] |
| 226. | Methyl ganoderate AM | - | G. lucidum (fruit bodies) |
[65] |
| 227. | Ganoderic aldehyde A | - | G. lucidum (fruit bodies) |
[65] |
| 228. | Ganoderenic acid K | - | G. lucidum (fruit bodies) |
[65] |
| 229. | Ganoderenic acid E | - | G. lucidum (fruit bodies) |
[65] |
| 230. | Elfvingic acid A | - | G. lucidum (fruit bodies) |
[65] |
| 231. | 12β-Acetoxy-3β,7β-dihydroxy-11,15,23-trioxo-5α-lanosta-8,20-dien-26-oic acid | - | G. lucidum (fruit bodies) |
[65] |
| 232. | Methyl ganolucidate C | - | G. lucidum (fruit bodies) |
[65] |
| 233. | Ganolucidic acid C | - | G. lucidum (fruit bodies) |
[65] |
| 234. | Ganoderic acid C2 | - | G. lucidum (fruit bodies /spore) |
[65] |
| 235. | Ganodrol A | Moderately inhibits FAAH (Inhibition rate in between 50–60%) | G. lucidum | [116][128] |
| 236. | Ganodrol C | Moderately inhibits FAAH (Inhibition rate in between 50–60%) | G. lucidum | [116][128] |
| 237. | Ganodrol D | Moderately inhibits FAAH (Inhibition rate in between 30–40%) | G. lucidum | [116][128] |
| 238. | Ganoderic acid XL5 | Cytotoxicity against human tumor cell lines—NE | G. theaecolum | [222][236] |
| 239. | Methyl gibbosate M | Anti-adipogenesis activity—NE | G. applanatum | [51] |
| 240. | Methyl ganoapplate E | Anti-adipogenesis activity—NE | G. applanatum | [51] |
| 241. | Applandiketone A | - | G. applanatum | [223][237] |
| 242. | Applandiketone B | Significant inhibitory effect against NO production in LPS-induced RAW264.7 cells (IC50 = 20.65 µM) |
G. applanatum | [223][237] |
| 243. | 15α-Acetoxy-3α-hydroxylanota- 8,24-dien-26-oic |
- | G. capense | [224][238] |
| 244. | Ganoderterpene A | Strongly suppressed NO generation in BV-2 microglial cells treated with lipopolysaccharide (LPS) (IC50 = 7.15 µM), significantly suppressed the activation of MAPK and TLR-4/NF-κB signaling pathways, effectively improved the LPS-induced mitochondrial membrane potential and apoptosis | G. lucidum | [225][239] |
| 245. | Ganodeweberiol G | Significant α-glucosidase inhibitory activity (IC50 = 165.9 µM) | G. weberianum | [77] |
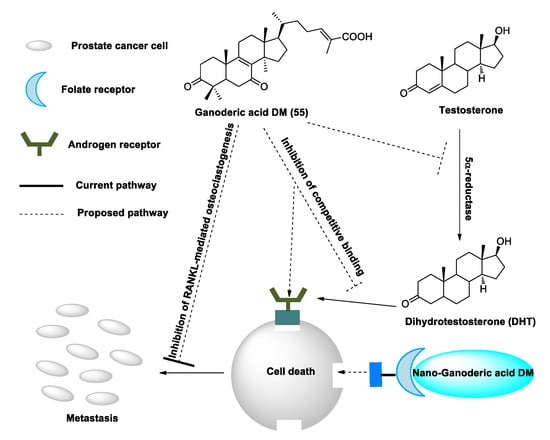
Figure 3. Suggested mechanisms by which ganoderic acid DM (GA-DM) may inhibit prostate cancer cell proliferation and metastasis.
Figure 4
).
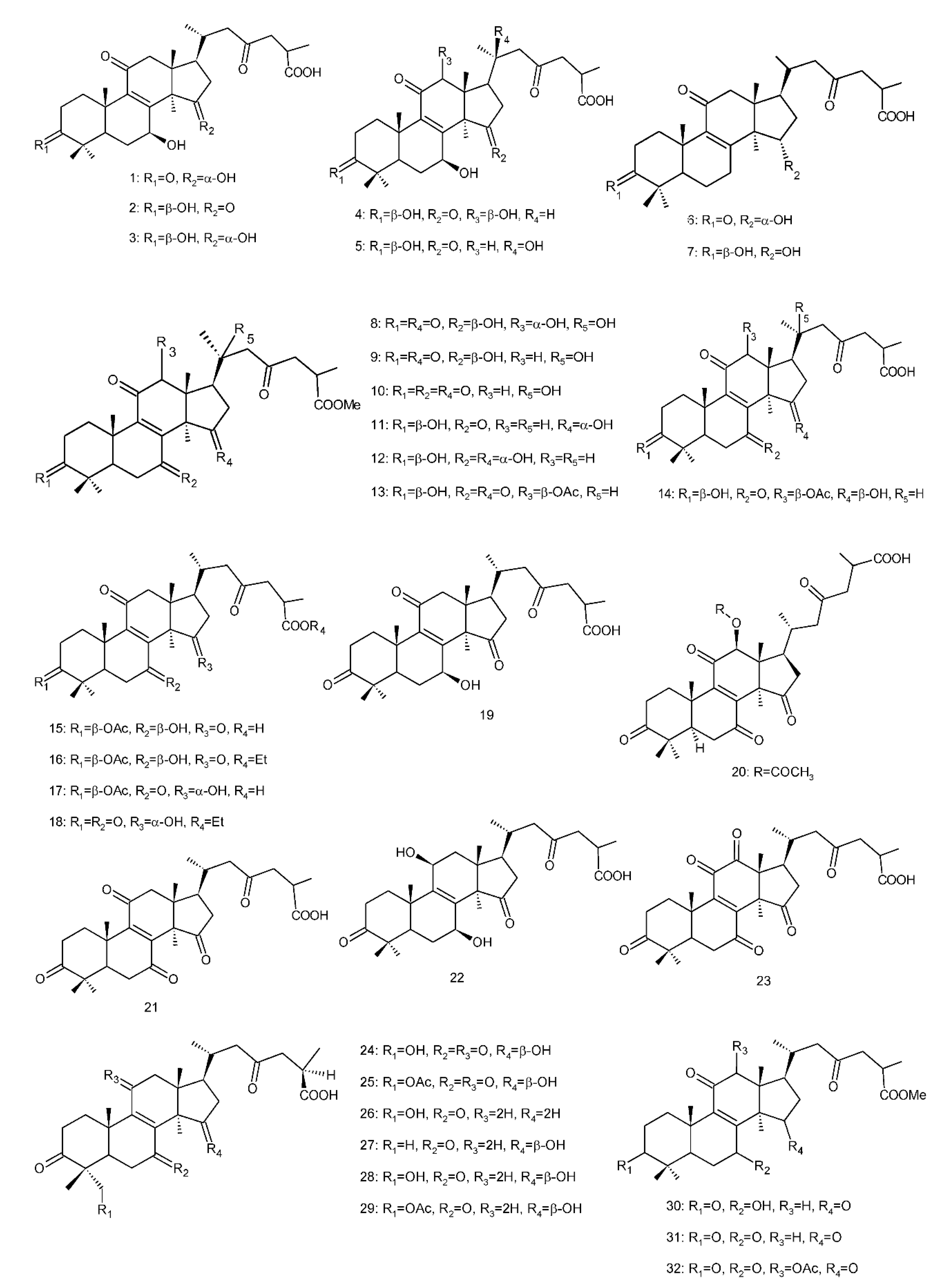
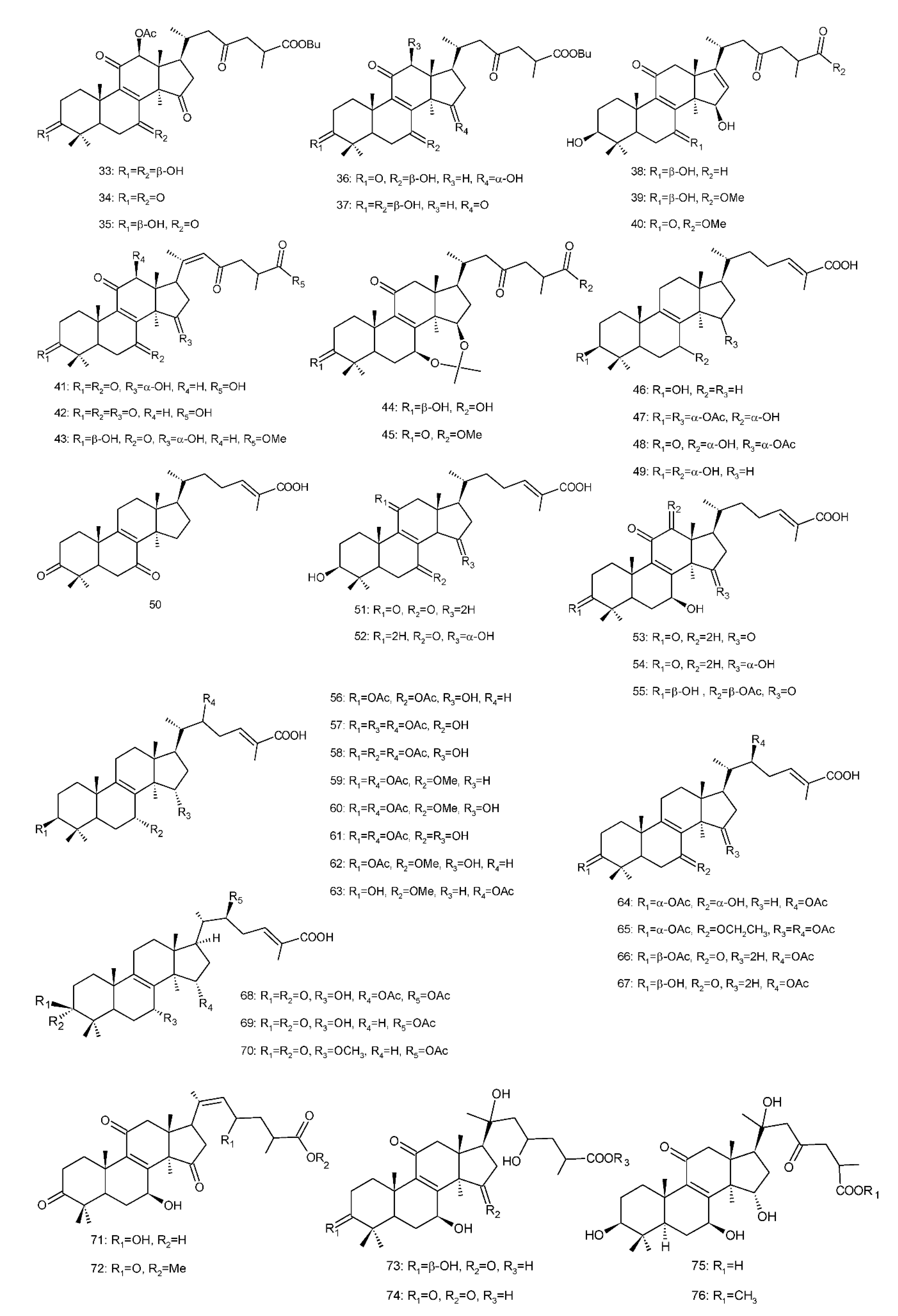
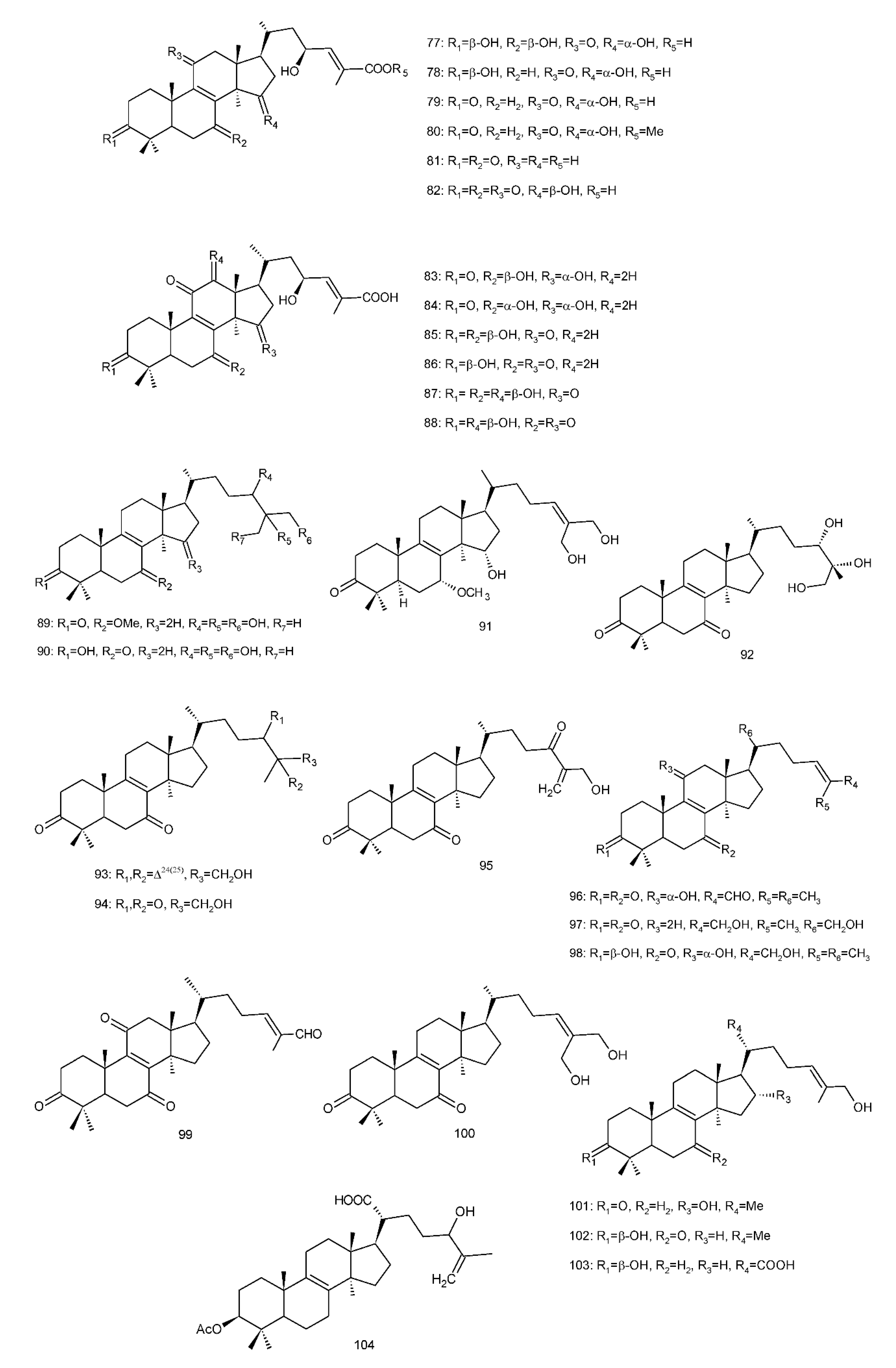
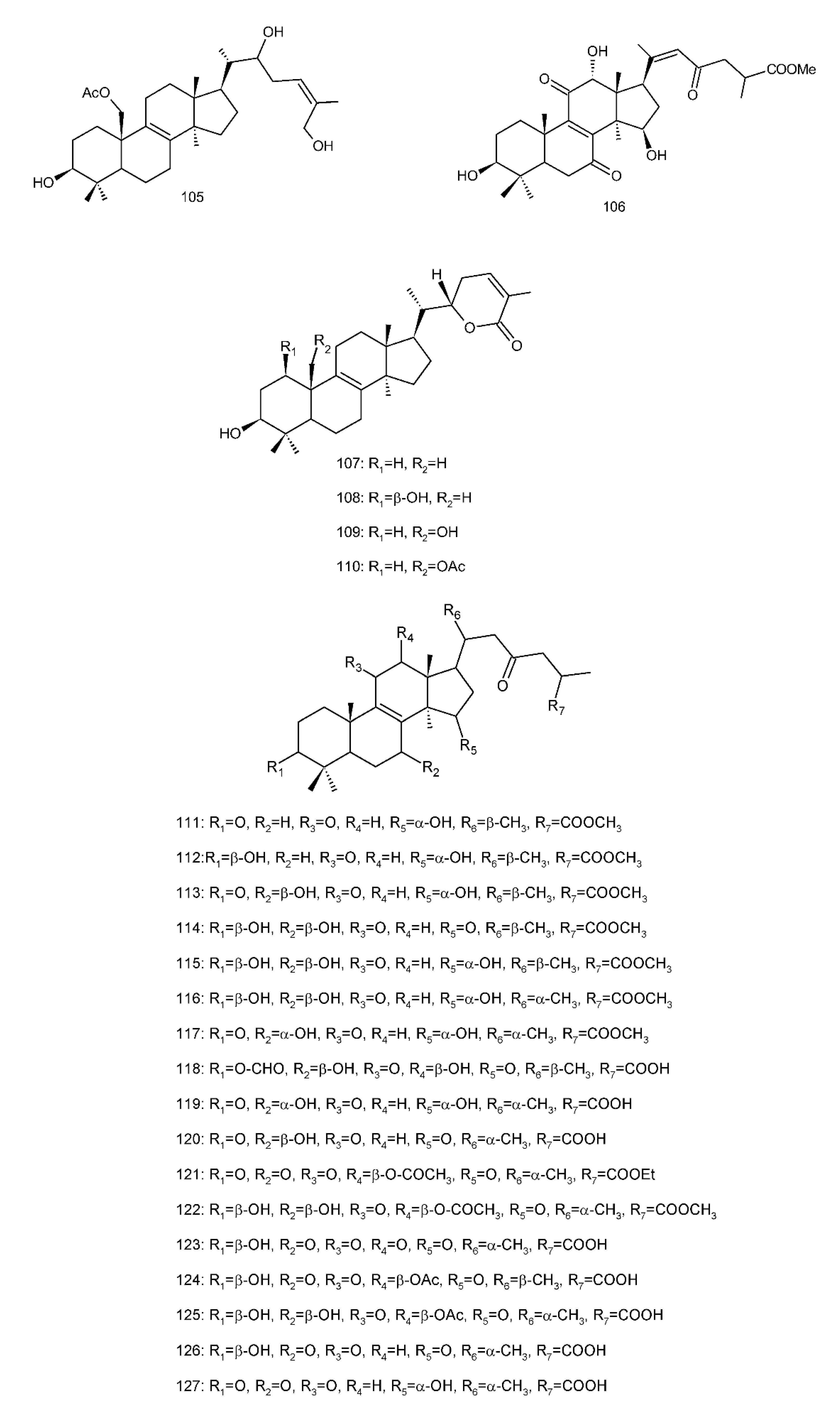
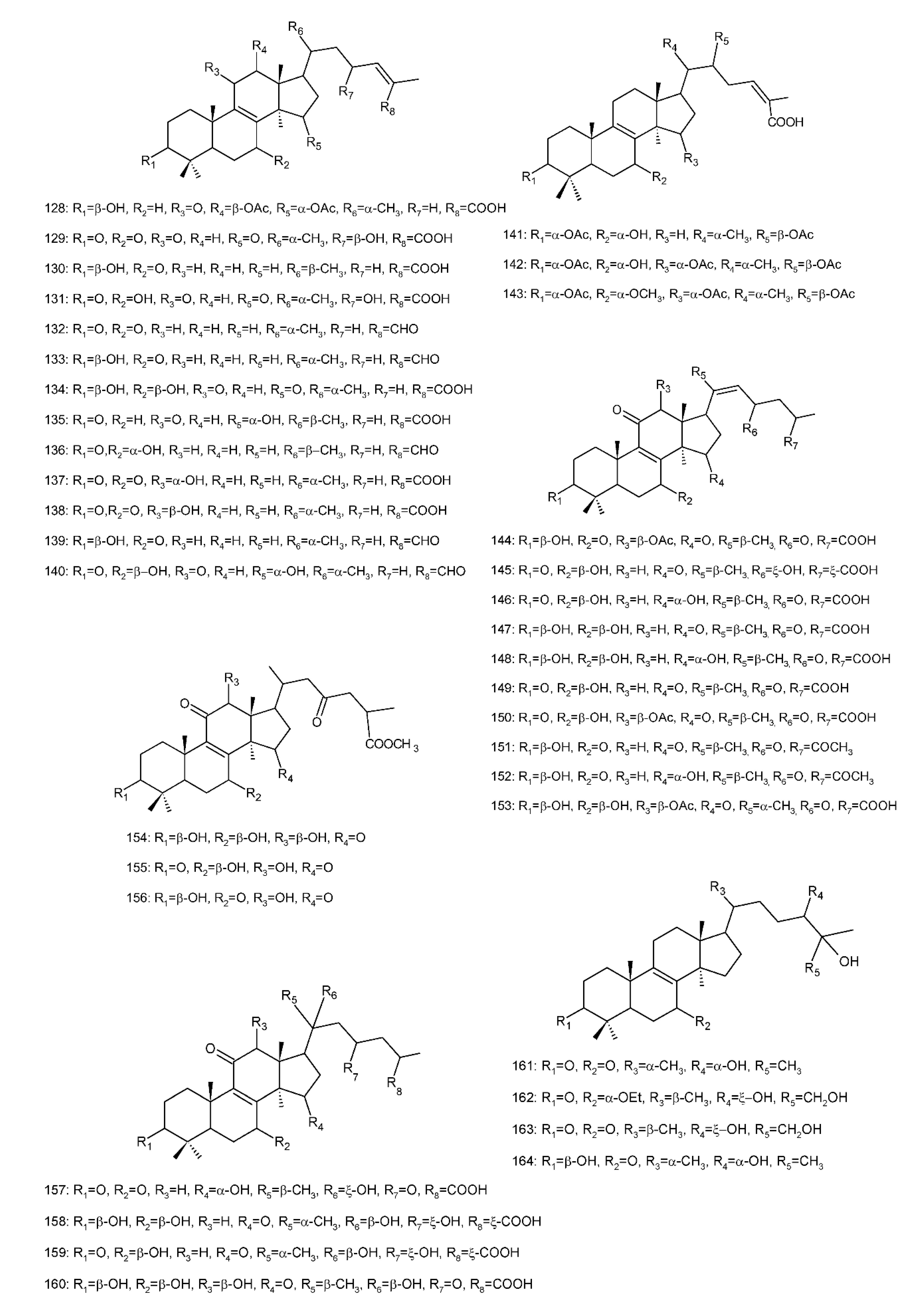
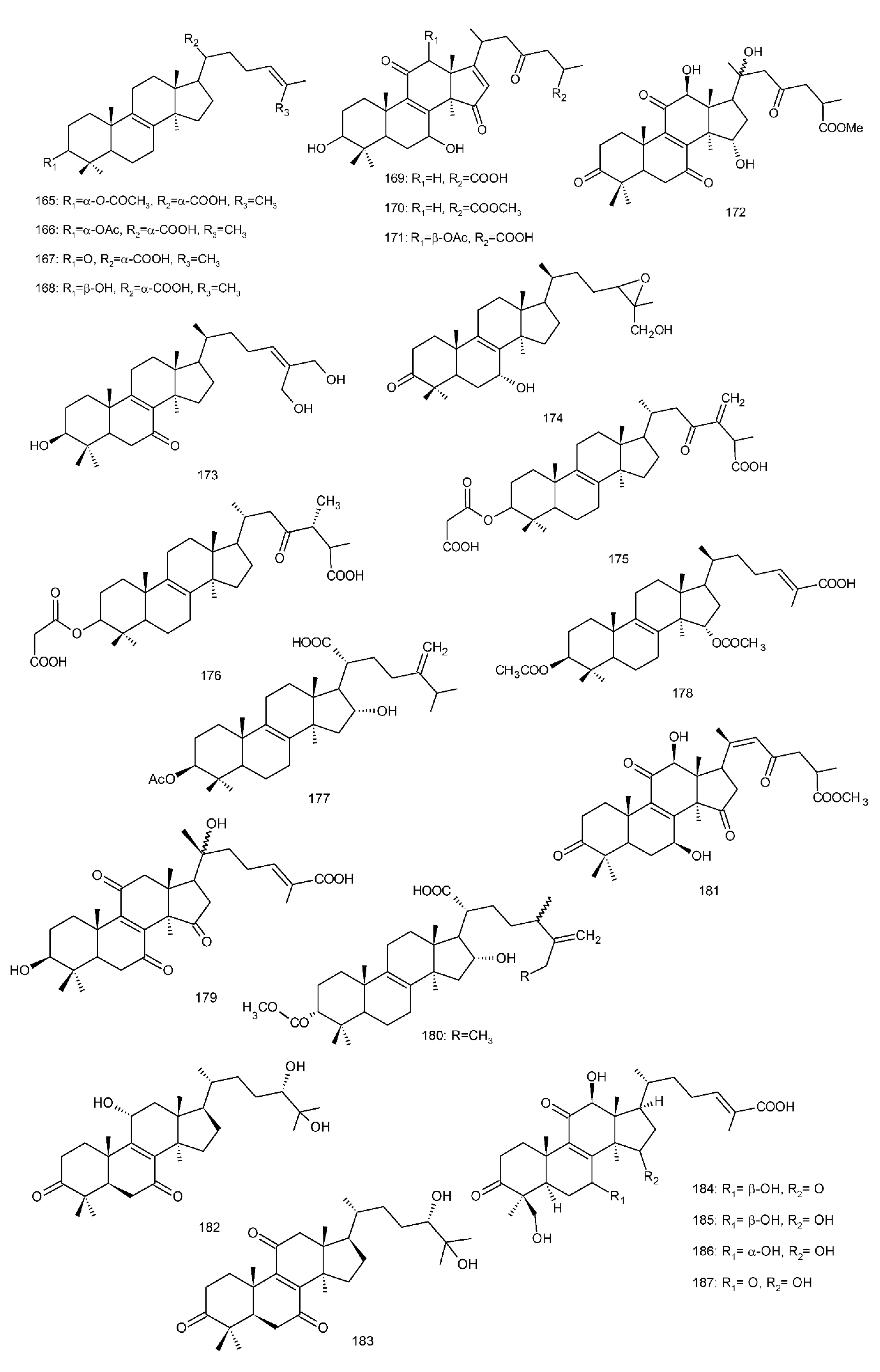
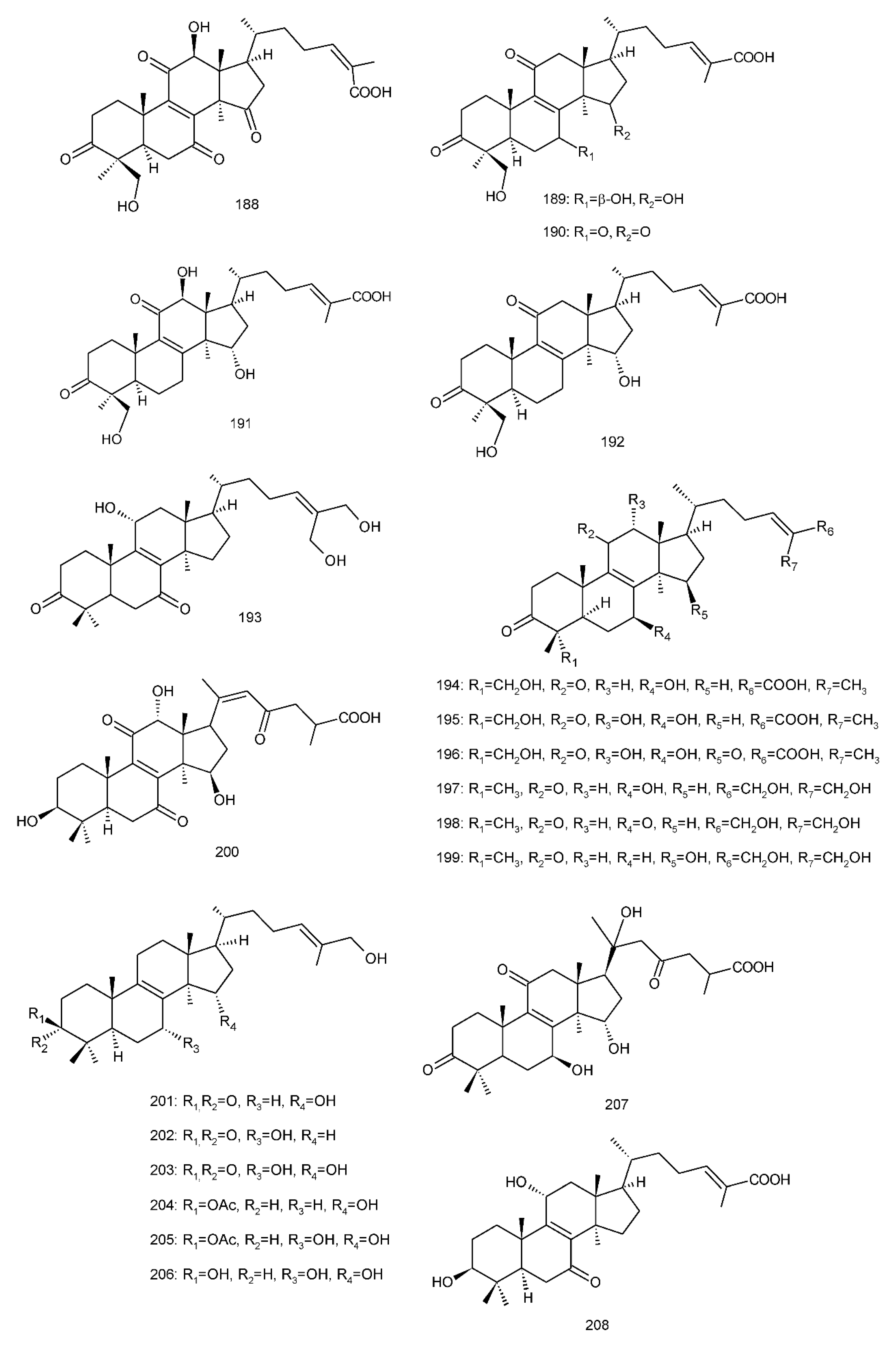
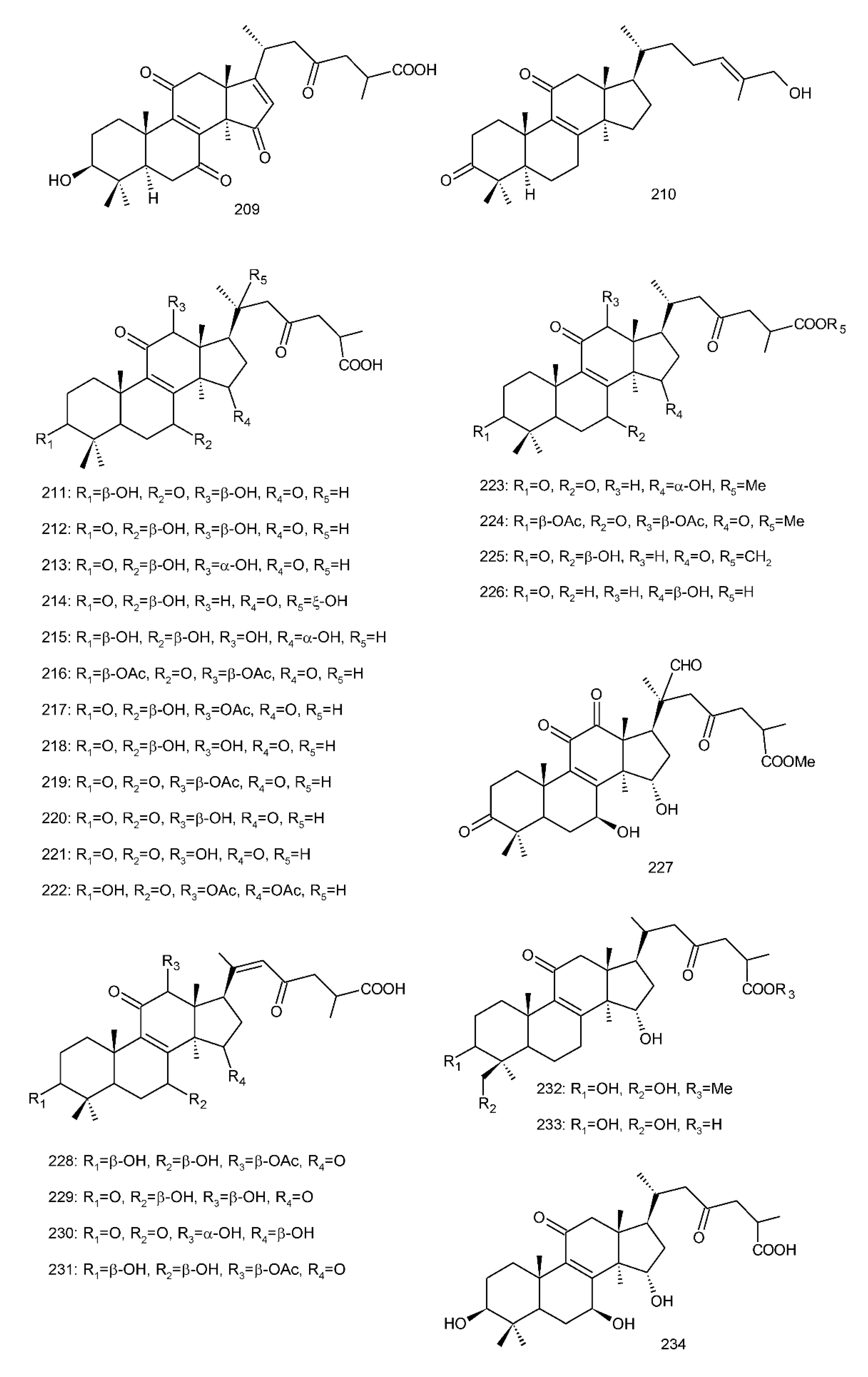
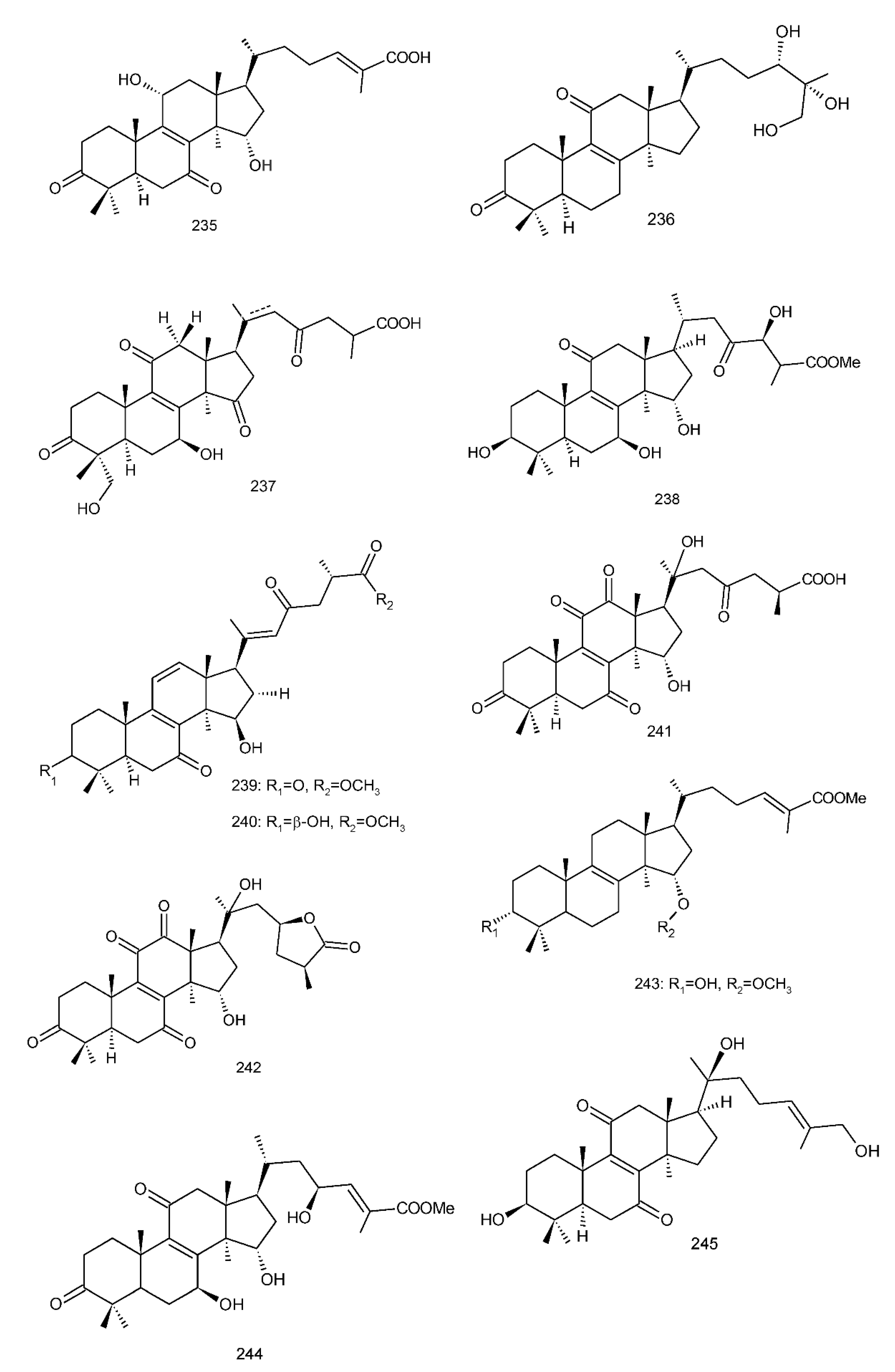
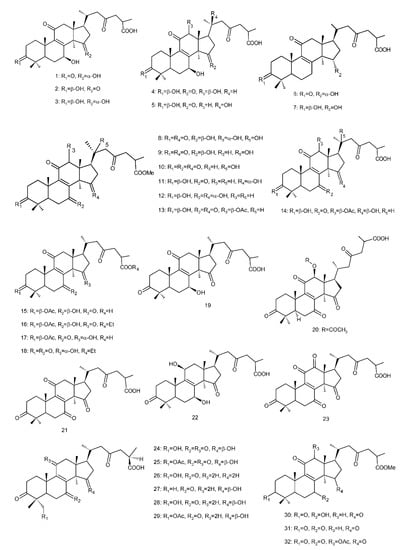
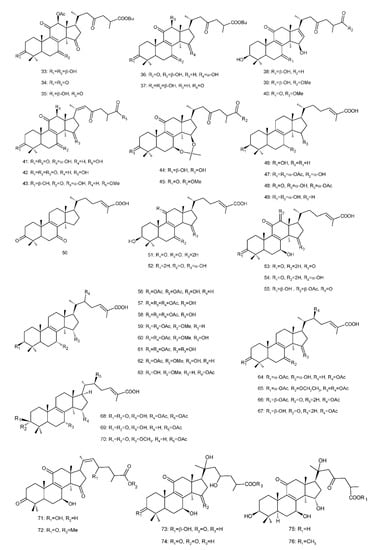
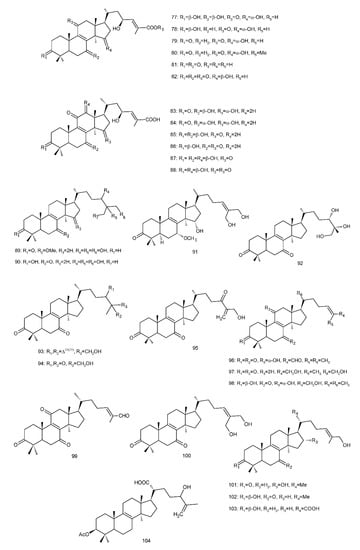
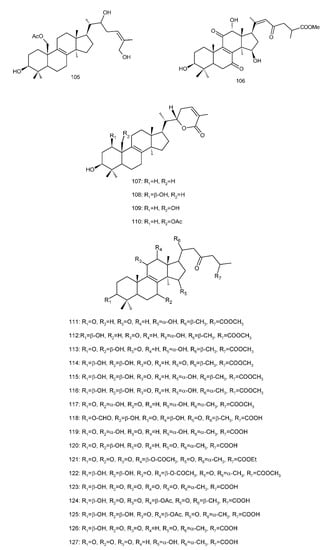

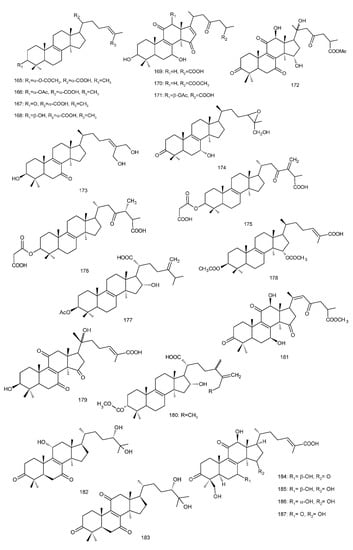
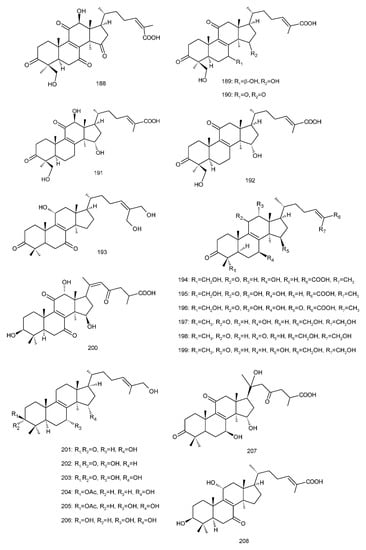
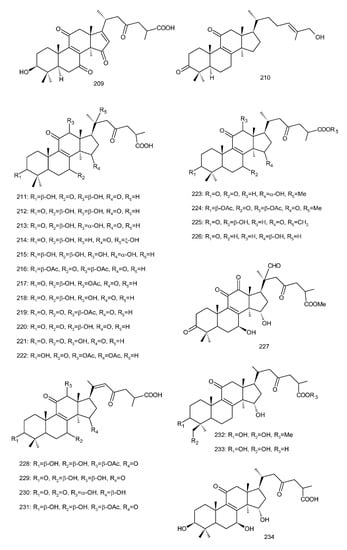
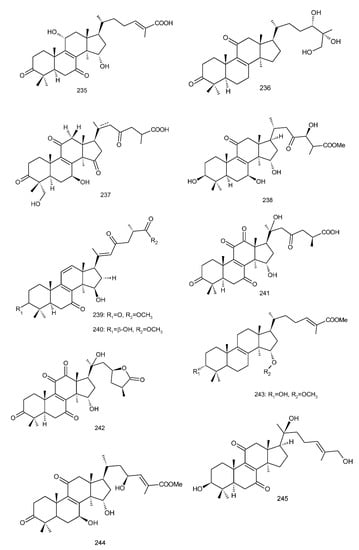
Figure 4. Chemical structures of several 8,9-double-bond triterpenoids (1–245).
