You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Mark Andreas Kluth.
The ATP-binding cassette superfamily member ABCB5 identifies a subset of skin-resident mesenchymal stem cells (MSCs) that exhibit potent immunomodulatory and wound healing-promoting capacities along with superior homing ability. The ABCB5+ MSCs can be easily accessed from discarded skin samples, expanded, and delivered as a highly homogenous medicinal product with standardized potency.
- ABCB5
- advanced-therapy medicinal product
- angiogenesis
- cell therapy
- homing
- immunomodulation
- mesenchymal stem cells
- potency assays
- product safety
1. Biological Properties of ABCB5+ MSCs
Immunostaining of healthy human skin sections revealed dermal ABCB5+ cell subsets to reside in the reticular dermis at frequencies ranging from 1.5% to 4.0% of dermal cells [1][2]. These cells are frequently confined to a perivascular endogenous niche in close association with CD31+ endothelial cells or found dispersed within the interfollicular dermis independent of hair follicles [2].
The ABCB5+ cells derived from enzymatically digested skin and separated by multiple rounds of magnetic-bead cell sorting using an anti-ABCB5 monoclonal antibody [3] are plastic-adherent, display a spindle-like, fibroblastoid cell morphology, exhibit a typical mesenchymal-lineage marker expression pattern [1][2] (summarized in Table 1), and demonstrate a consistent and significantly increased potential for in vitro adipogenic, osteogenic, and chondrogenic lineage differentiation as compared with donor-matched ABCB5– dermal fibroblasts [2]. In another contrast to ABCB5− fractions, ABCB5+ dermal cells were shown to give rise to single cell-derived colonies, with clonal colonies generated from single cells again displaying clonogenic growth. Remarkably, 76% of these self-renewed clones maintained their trilineage differentiation potential, and all self-renewed clones could differentiate into at least one mesenchymal lineage [2]. In addition, studies in murine muscle injury and skin wound models have indicated in vivo myogenic and endothelial differentiation potential, respectively [4][5].
Table 1.
Cell marker expression pattern of ABCB5
+
MSCs.
| Cell Marker | Typically Expressed by | Expression by ABCB5+ MSCs | Detection Method | References |
|---|---|---|---|---|
| CD73 | MSCs | Yes | FCM | [1][2] |
| CD90 | MSCs | Yes | FCM | [2] |
| CD105 | MSCs | Yes | FCM | [1][2] |
| CD29 | MSCs | Yes | FCM | [1] |
| CD44 | MSCs | Yes | FCM | [1] |
| CD49e | MSCs | Yes | FCM | [1] |
| CD166 | MSCs | Yes | FCM | [1] |
| CD14 | Monocytes/macrophages | No | FCM | [2] |
| CD20 | B lymphocytes | No | FCM | [2] |
| CD34 | Hematopoietic-lineage cells, dendritic cells | No | IF | [1] |
| FCM | [1][2] | |||
| CD45 | Hematopoietic-lineage cells | No | FCM | [1][2] |
| CD31 | Endothelial-lineage cells | No | IF | [1][2] |
| FCM | [1] | |||
| NG2 | Pericytes | No | IF | [2] |
| CD318 | Epithelial cells | No | FCM | [2] |
| MelanA | Melanocytic cells | No | IF | [6] |
| FCM | [2] | |||
| CD133 | Cancer stem cells, malignant melanoma cells | No | IF | [6] |
| FCM | [2] | |||
| LGR5 | Hair follicle stem cells | No | IF | [6] |
| LNGFR/CD271 | Neuro-ectodermal skin-derived precursors | No | FCM | [2] |
| SSEA-4 | Stem cells | Yes | IF, FCM | [2] |
| SOX2 | Stem cells | Yes | IF | [2] |
| POU5F1/Oct4 | Stem cells | Yes | IF | [2] |
| DPP-4/CD26 | Upper-lineage fibroblasts | Yes | IF | [2] |
| PRDM1/BLIMP-1 | Upper-lineage fibroblasts | Yes | IF | [2] |
| α-SMA | Lower-lineage fibroblasts | No | IF | [2] |
BLIMP-1—B lymphocyte-induced maturation protein 1; CD—cluster of differentiation; DPP-4—dipeptidyl-peptidase 4; FCM—flow cytometry; IF immunofluorescence staining; LGR5—leucine-rich G protein-coupled receptor 5; LNGFR—low-affinity nerve growth factor receptor; NG2—neural/glial antigen 2; Oct4—octamer-binding transcription factor 4; POU5F1—POU domain class 5 transcription factor 1; PRDM1—PR domain zinc finger protein 1; α-SMA—α-smooth muscle actin; SOX2—Sex-determining region Y box 2; SSEA—stage-specific embryonic antigen 4.
Together, dermal ABCB5+ cells display morphological and functional properties similar to conventional mesenchymal stromal cells [7] and, in addition, possess self-renewal and differentiation capacity in vitro and in vivo, thereby meeting the criteria for classification as a mesenchymal stem cell (MSC) [8] and substantiating the conclusion that ABCB5+ identifies a MSC population amongst dermal mesenchymal stromal cells.
2. Physiological Functions of ABCB5+ MSCs
2.1. Stem Cell Integrity and Quiescence
The expression of ABC transporters including ABCB5 by stem cells has predominantly been ascribed to their ability of preventing toxic compounds from entering the cell and effluxing secondary metabolites out of the cell. This is thought to confer protection to these long-lived cell subsets against xenobiotics, thereby contributing to maintenance of stem cell integrity [9][10]. In addition to these protective functions, ABCB5 expressed on dermal MSCs appears to be involved in cell cycle regulation. Specifically, blocking ABCB5 function on ABCB5+ MSCs in vitro, using a neutralizing anti-ABCB5 antibody, resulted in an increase in actively proliferating ABCB5+ MSCs [11]. This finding supports a role for ABCB5 as a mediator of stem cell quiescence of ABCB5+ MSCs, as was previously demonstrated for other ABCB5-expressing stem cell types such as limbal stem cells and cancer stem cells [12][13].2.2. Cutaneous Regeneration and Wound Healing
Indications for a role of ABCB5+ MSCs in normal skin regeneration have come from age-related changes in numbers, niche preferences and differentiation capacities of dermal ABCB5+ MSCs, which correlate with the reduced regenerative capacity of the aging skin. Specifically, immunostaining analyses of a series of human skin specimens revealed a significant decline of dermal ABCB5+ MSC frequency from about 3.2% of all dermal cells in young individuals (≤20 years) to about 1.6% in individuals aged above 70 years [6]. At the level of gene expression, age-dependent increases in the expression of the DNA double-strand break marker γH2AX, of various genes involved in the nucleotide excision repair pathway and of pro-apoptotic genes including p73 suggest that accumulation of DNA damage and enhanced apoptosis represent significant challenges to the integrity and numbers of ABCB5+ MSCs in old individuals [14].
The age-dependent decline in ABCB5+ MSCs was shown to be concomitant with a change in niche preference of ABCB5+ MSCs from a predominant (75%) perivascular localization in close association to endothelial cells of small vessels in the skin of young individuals to a predominant (90%) interfollicular localization in the skin of old individuals [6]. Both the decrease in number and the perivascular niche preference of ABCB5+ MSCs coincided with a decrease of perivascular osteopontin, an extracellular matrix (ECM) component provided by perivascular neural glial antigen 2-expressing niche pericytes [15]. Interestingly, in an osteopontin-depleted mouse model, numbers of dermal ABCB5+ MSCs were even lower than in aged wild-type mice, pointing to a pivotal role of osteopontin in the regulation of dermal ABCB5+ MSC biology [15]. Furthermore, ABCB5+ MSCs of older individuals showed a gradual decrease in the percentages of cells expressing the stem cell markers stage-specific embryonic antigen 4 and sex-determining region Y box 2 [15], along with an enhanced adipogenic and markedly reduced osteogenic and chondrogenic differentiation potential as compared with ABCB5+ MSCs of young individuals [14][15].
Collectively, a robust decrease in ABCB5+ MSCs together with distinct changes in niche preference and differentiation capacity may contribute to the reduced regenerative capacity of the aged skin, in turn suggesting critical roles for ABCB5+ MSCs in normal skin homeostasis and physiological regeneration [6][11][14]. Moreover, ABCB5 has been found to be functionally involved in cutaneous wound healing through molecular regulation of a proangiogenic pAkt/hypoxia-inducible factor (HIF)-1α/vascular endothelial growth factor (VEGF) cascade, based on delayed wound closure, increased inflammatory stroma thickness and aberrant angiogenesis associated with impaired pAkt/HIF-1α/VEGF signaling observed in Abcb5-knockout as compared with wildtype mice [16].
3. Therapeutic Use
3.1. Feasibility of Allogeneic Use
Mesenchymal stem cell therapy requires safe and efficacious administration of the cell product. First attempts of therapeutic MSC administration used autologous MSCs, derived from the patients themselves, and autologous MSCs are still used in most clinical trials [17]. For the first clinical trial of ABCB5+ MSCs, the cells were derived from small skin biopsies of the patients and expanded ex vivo, before being topically applied onto chronic, standard therapy-refractory venous ulcers (CVUs) [5]. While the clinical outcome of a median 63% wound size reduction at 12 weeks from baseline, also associated with early pain reduction, substantiated the view that autologous ABCB5+ MSCs could deliver a clinically relevant wound closure strategy, the autologous approach emerged associated with several drawbacks owing to the labor- and time-consuming manufacturing process, which was carried out for each patient individually. This was associated with comparably high costs and weeks- or monthslong delays until the treatment could be started, and generated only limited numbers of cells, which neither enabled treatment of larger wounds [5] nor would allow for higher cell number-requiring potential systemic therapy approaches. In addition, candidate patients for MSC therapies are often of advanced age and frequently affected by comorbidities. Unfortunately, age and disease state can negatively affect the number and functionality of MSCs [18]. The ABCB5+ MSCs, as outlined above, frequently underlie age-dependent declines in the number of cells present in situ [6][16] and can accumulate DNA damage and enhanced apoptosis [14] as well as changes in the differentiation potential [14][15]. Moreover, diabetes, at least in mice, is associated with reduced numbers of ABCB5+ MSCs along with profound changes in the dermal stromal niche [19]. These hurdles associated with the use of autologous MSCs prompted additional research on allogeneic treatment strategies. In the allogeneic setting, ABCB5+ MSCs can be obtained from young, healthy donors and manufactured as a ready-to-use product of consistent quality [20]. The generally low immunogenicity of resting MSCs, owing to only low or modest expression of MHC class I molecules and lack of MHC class II and co-stimulatory molecules required for full T-cell activation [21][22][23], and their immunosuppressing capacities contribute to a lower immune rejection response elicited by transplanted allogeneic MSCs compared to other cell types [24][25]. Therefore, although previous studies that controlled for MHC haplotype of donors and recipients suggested that MHC-mismatched MSCs may not be immune-privileged [25][26][27] and that about 11.5% of patients may develop donor-specific antibodies upon administration of allogenous MSCs [28], the transplantation of allogeneic MSCs typically requires no immunosuppressive treatment of the recipient. A body of studies that directly compared immune-modulatory and regenerative efficacies of allogeneic vs. autologous MSCs in immunocompetent animals or in humans have confirmed that allogeneic MSCs are not inferior to autologous MSCs [29][30][31][32][33][34][35]. This applies also to ABCB5+ MSCs: Comparison of clinical trial results using either autologous, patient-derived or allogeneic, donor-derived ABCB5+ MSCs to treat chronic, treatment-refractory CVUs reveals that allogeneic treatment achieved a median wound size reduction from baseline at 12 weeks of 76% (full analysis set) and 78% (per-protocol set) with 20% and 22%, respectively, of wounds fully closed [36], as compared with 63% wound size reduction and 17% full wound closure rate in the autologous setting [5]. Moreover, in fully MHC-mismatched cardiac allotransplantation models, allogeneic (donor-strain or third party-strain) ABCB5+ MSCs profoundly prolonged graft survival, whereas treatment with syngeneic (recipient-strain) ABCB5+ MSCs showed only modest prolongation of graft survival, indicating that prolonged enhancement of cardiac allograft survival by treatment with ABCB5+ MSCs even depends on MSC-dependent allogeneic tolerogenic stimulation [1].3.2. Homing and Engraftment
When tissues are damaged, endogenous MSCs migrate to the site of injury to locally trigger mechanisms that promote regeneration [37]. The resultant rationale behind therapeutic application of MSCs is that transplanted MSCs would also migrate and home to the damaged tissues [38] (Figure 1).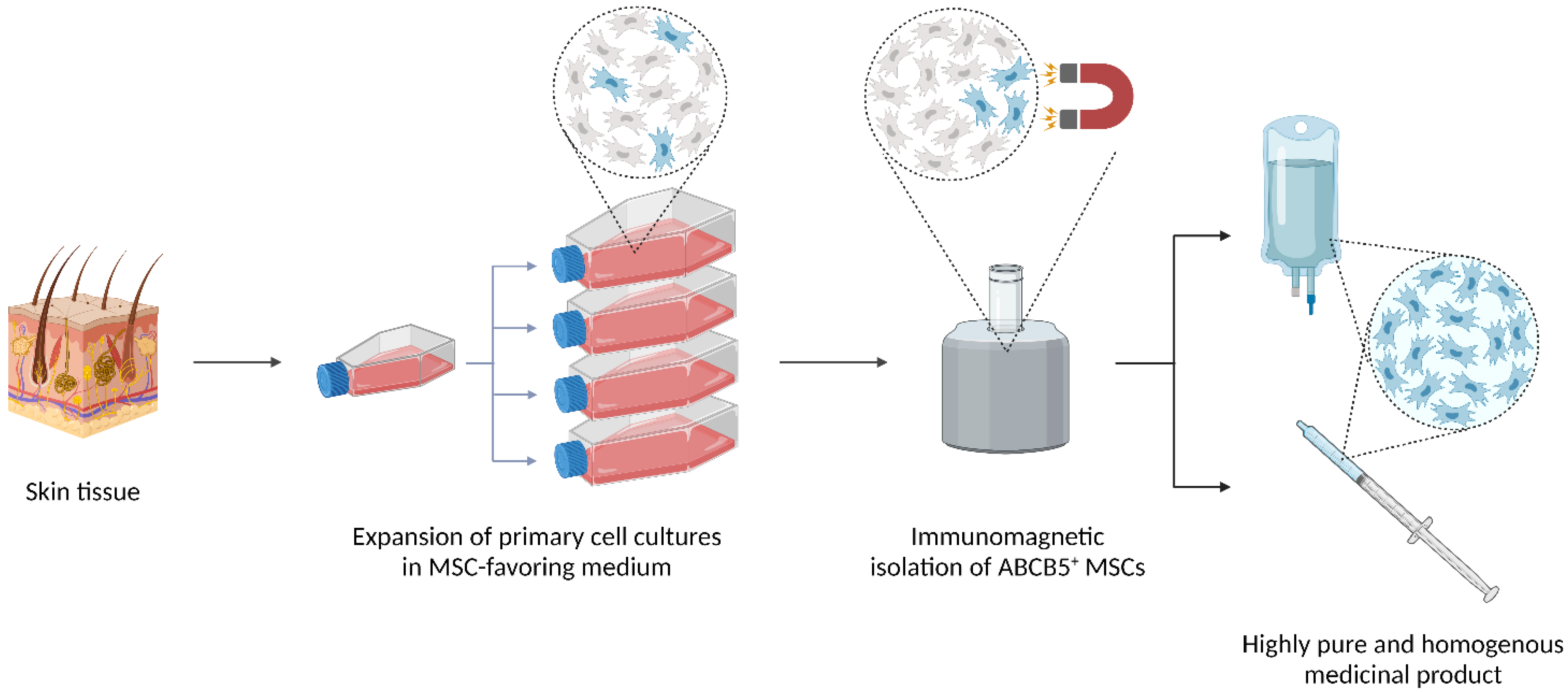
Figure 1. Clinical use and effects of ABCB5+ MSCs. Upon topical or systemic administration, ABCB5+ MSCs migrate and home to injured, inflamed and/or hypoxic tissues to exert anti-inflammatory, pro-angiogenic and trophic responses that facilitate wound healing and enhance skin integrity. The mode-of-action pathways that are utilized for routine potency testing of the MSC-based medicinal product are highlighted in yellow. HIF-1α—hypoxia-inducible factor 1α; HOXA3—homeobox A3; M1—M1 macrophage; M2—M2 macrophage; VCAM-1—vascular adhesion molecule 1. Created with BioRender.com.
3.3. Product Quality
3.3.1. Homogeneity
The ATMPs based on living cells are inherently prone to heterogeneity because the cells’ gene and expression profiles may vary considerably depending on variations in donor characteristics as well as methods and conditions of cell expansion, cell isolation and product formulation [47][48][49][50]. In the ABCB5+ MSC manufacturing process, careful donor selection utilizing rigorous in-/exclusion criteria regarding age and health state, strict definitions of all production steps and in-process controls at every stage of production [20], and, not at least, the use of a unique cell surface marker (ABCB5) specifically selecting for a reproducibly potent MSC subpopulation [1][2] are critical tools to minimize potential fluctuations. Indeed, during process validation, comparative gene expression analyses revealed only rare variations between donors as well as between different passages within donors, which indicates high intra- and inter-donor homogeneity of the expanded cells [5]. As evaluated in periodic GMP product quality reviews [20], homogeneity of GMP-compliantly manufactured ABCB5+ MSCs is reliably achieved by close monitoring and continuous refinement of each process step and mandatory release tests ensuring identity, purity, and biological activity of the produced cells. Specified, validated acceptance criteria guarantee that each released cell batch contains ≥90% ABCB5+ cells (actually 97.7 ± 1.9%) and ≥90% CD90+ cells (actually 99.5 ± 0.5%) at ≥90% cell vitality (actually 98.6 ± 0.6%) and ≥90% cell viability (actually 99.5 ± 0.6%) (means ± SD of 66 cell batches derived from seven donors [20].3.3.2. Potency
A vital component of the quality assessment of cell therapy products is the evaluation of the cells’ actual biological functionality using appropriate tests that predict their clinical effectiveness [51][52]. A validated potency assay with predefined acceptance criteria guarantees that the product exerts a certain intended effect at a specific dose and ensures that the treated patient receives a potent therapy product [53]. Considering that ABCB5+ MSCs exert their therapeutic effects through multiple pathways induced upon interaction with the host microenvironment, three potency assays have been integrated into the manufacturing process to reflect the most clinically relevant biological modes of action of ABCB5+ MSCs:3.4. Safety
3.4.1. Product Safety
Ex vivo expansion entails enhanced cell division rates in an artificial environment lacking the physiological mechanisms of negative selection and clearance of altered cells that are active in the whole organism [55]. Therefore, in order to facilitate early detection of signals of non-physiological growth behavior during cell expansion that might indicate potential deleterious effects on cell biology including tumorigenic transformation, several in-process controls monitoring cell morphology, contact inhibition, time between passages and cell cycle phase distribution are vital components of the routine ABCB5+ MSC manufacturing process [20]. To ensure sterility and purity of the manufactured cell products, specified validated control procedures guard, at each production step, against potential contamination of the cultures, cellular intermediate and final products with infectious agents (including aerobic and anaerobic bacteria and fungi, mycoplasma, and endotoxins) and residual impurities [20]. Only cultures and batches that meet specified release criteria ensuring product sterility and purity are released for further processing, cryostorage, or delivery [20].3.4.2. Preclinical Safety Profile
The preclinical safety profile of GMP-compliantly produced, reconstituted (ready-to-use) ABCB5+ MSCs has been determined in a Good Laboratory Practice-conforming preclinical in vivo study program following the recommendations of the European Medicines Agency [51] addressing all relevant aspects of biosafety [56]. All studies were performed in severely immunocompromised (NOD scid or NSG) mice to prevent rejection of the administered cells in the xenogeneic host. In these mice, subcutaneously injected ABCB5+ MSCs did not significantly migrate to other tissues and organs, indicating confinement of the cells to the application site following local administration. Systemic (intravenous) infusion at a concentration intended for use in clinical trials (1 × 107 cells/mL [20][57]) was well tolerated without any clinical signs or mortality indicative of clinically relevant pulmonary embolus formation by cells mechanically entrapped in the lungs. Repeated subcutaneous or intravenous injections neither provoked any signs of human cell-related tumor development, ectopic tissue formation or micrometastases, nor did it elicit any signal indicative of ABCB5+ MSC-related toxicity regarding mortality, clinical signs, body weight development, food consumption, ophthalmological examination, urine analysis, hematology, blood chemistry, blood coagulation, and macro-pathological and histopathological examination. Together, these data demonstrated a favorable biosafety profile of GMP-manufactured ABCB5+ MSCs in terms of distribution to non-target tissues, toxicity, ectopic tissue formation, or tumor development [56]. In intramuscular local-tolerance studies, injection of ABCB5+ MSCs into the thigh muscles of NOG mice resulted in a slight increase in microscopically detectable inflammatory and detectable processes in the muscular tissue when compared to vehicle-treated animals at 1 week, whereas at 4 weeks no differences between cell- and vehicle-injected thigh muscles were detectable [56]. Several lines of evidence of liver safety of ABCB5+ MSCs have come from studies investigating the effects of ABCB5+ MSCs, delivered to the liver via the intrasplenic route to bypass pulmonary entrapment, in an Pfp/Rag2−/− immunodeficient mouse model of liver regeneration after partial (one third) liver resection [58][59]. In this model, GMP-manufactured ABCB5+ MSCs did not augment the increase in serum transaminases that occurred following partial hepatectomy over controls at week 7 after MSC application [59]. In addition, ABCB5+ MSC did not impact on physiological lipid accumulation, hepatocyte proliferation rate, physiological zonal distribution of metabolism markers (i.e., periportal expression of E-cadherin, and perivenous expression of glutamine synthetase and cytochrome P450 2E1) during seven weeks of liver regeneration [59]. Moreover, ABCB5+ MSCs did not induce toxicity regarding liver fibrosis (as assessed by collagen deposition and expression of Timp1, the gene encoding tissue inhibitor of metalloproteinases 1), inflammation (as assessed by expression of the pro-inflammatory cytokines Il1B and Il6 in liver tissue) or hepatocellular destruction (as assessed by expression of caspases 3 and 9 in liver tissue) as compared with vehicle-treated animals at week 7 after MSC application [58].3.4.3. Safety Data from Clinical Trials
At present, the clinical safety of allogenic ABCB5+ MSCs has been investigated in three completed phase I/IIa clinical trials [36][54][57]. Overall, 54 adult patients presenting with chronic wounds received, in total, 92 topical doses of 1–2 × 106 ABCB5+ MSCs per cm2 wound surface area [36][54], and 16 patients aged 4–36 years suffering from RDEB received 46 intravenous infusions of 2 × 106 ABCB5+ MSCs per kg body weight in total [57]. Treatment-related adverse events were reported with 3 of 92 (3.3%) topical and 3 of 46 (6.5%) of intravenous applications. Treatment-related AEs reported from topical treatment were related to the treated wounds and were of mild or moderate severity. None of these events were serious, and all resolved without sequelae [36]. Treatment-related AEs reported from intravenous treatment were one mild lymphadenopathy and two severe hypersensitivity events, which may have resulted from immunological sensitization following the previous ABCB5+ MSC infusion. These latter two events were considered serious; however, the affected patients recovered without sequelae shortly after withdrawal of treatment [57]. Given that the risk of hypersensitivity reactions can likely be reduced in future treatments by monitoring potential induction of anti HLA antibodies and premedication with antihistamines, the Trial Data Monitoring Committee evaluated the potential risk of hypersensitivity reactions as being justified by the anticipated treatment benefits for RDEB patients [57]. Together, the clinical experience to date indicates a favorable safety profile of the cell product with few mild and/or manageable adverse events.References
- Schatton, T.; Yang, J.; Kleffel, S.; Uehara, M.; Barthel, S.R.; Schlapbach, C.; Zhan, Q.; Dudeney, S.; Mueller, H.; Lee, N.; et al.et al. ABCB5 Identifies Immunoregulatory Dermal Cells. Cell Rep. 2015, 12, 1564-1574, 10.1016/j.celrep.2015.08.010.
- Vander Beken, S.; de Vries, J.C.; Meier-Schiesser, B.; Meyer, P.; Jiang, D.; Sindrilaru, A.; Ferreira, F.F.; Hainzl, A.; Schatz, S.; Muschhammer, J.; et al.et al. Newly Defined ATP-Binding Cassette Subfamily B Member 5 Positive Dermal Mesenchymal Stem Cells Promote Healing of Chronic Iron-Overload Wounds via Secretion of Interleukin-1 Receptor Antagonist. Stem Cells 2019, 37, 1057-1074.
- Frank, N.Y.; Pendse, S.S.; Lapchak, P.H.; Margaryan, A.; Shlain, D.; Doeing, C.; Sayegh, M.H.; Frank, M.H.; Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J. Biol. Chem. 2003, 278, 47156-47165, 10.1074/jbc.M308700200.
- Kim, S.; Meier, B.; Schatton, T.; Wilson, B.; Zhan, Q.; Loh, Y.H.; Daley, G.Q.; Sayegh, M.H.; Ziouta, Y.; Ganss, C.; et al.et al. Identification of human ABCB5+ dermal progenitor cells with multipotent differentiation plasticity. J. Invest. Dermatol. 2010, 130 (Suppl. 1), S107, 10.1038/jid.2010.71.
- Kerstan, A.; Niebergall-Roth, E.; Esterlechner, J.; Schröder, H.M.; Gasser, M.; Waaga-Gasser, A.M.; Goebeler, M.; Rak, K.; Schrüfer, P.; Endres, S.; et al.et al. Ex vivo-expanded highly pure ABCB5(+) mesenchymal stromal cells as Good Manufacturing Practice-compliant autologous advanced therapy medicinal product for clinical use: process validation and first in-human data. Cytotherapy 2021, 23, 165-175, doi: 10.1016/j.jcyt.2020.08.012.
- de Vries, J.C.; Meier, B.; Jiang, D.; Frank, N.Y.; Vander Beken, S.; Ziouta, Y.; Kluth, A.; Ganss, C.; Frank, M.H.; Scharffetter-Kochanek, K.; et al. Towards further characterization of ABCB5+ mesenchymal stem cells in the ageing skin. Exp. Dermatol. 2015, 24, E5, doi: 10.1111/exd.12623.
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E.; et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315-317, doi: 10.1080/14653240600855905.
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L.; Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019-1024, doi:10.1016/j.jcyt.2019.08.002.
- Saeed, M.E.M.; Boulos, J.C.; Machel, K.; Andabili, N.; Marouni, T.; Roth, W.; Efferth, T.; Expression of the Stem Cell Marker ABCB5 in Normal and Tumor Tissues. In Vivo 2022, 36, 1651-1666, doi: 10.21873/invivo.12877.
- Frank, N.Y.; Frank, M.H.; ABCB5 gene amplification in human leukemia cells. Leuk. Res. 2009, 33, 1303-1305, doi: 10.1016/j.leukres.2009.04.035.
- de Vries, J.; Meier, B.; Vander Beken, S.; Jiang, D.; Frank, N.Y.; Kampilafkos, P.; Kluth, A.; Ganss, C.; Frank, M.H.; Scharffetter-Kochanek, K.; et al. ABCB5 is a stem cell cycle regulator in MSCs of the skin. Exp. Dermatol. 2016, 25, E46, doi: 10.1111/exd.12952.
- Ksander, B.R.; Kolovou, P.E.; Wilson, B.J.; Saab, K.R.; Guo, Q.; Ma, J.; McGuire, S.P.; Gregory, M.S.; Vincent, W.J.; Perez, V.L.; et al.et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 2014, 511, 353-357, doi: 10.1038/nature13426.
- Wilson, B.J.; Saab, K.R.; Ma, J.; Schatton, T.; Pütz, P.; Zhan, Q.; Murphy, G.F.; Gasser, M.; Waaga-Gasser, A.M.; Frank, N.Y.; et al.et al. ABCB5 maintains melanoma-initiating cells through a proinflammatory cytokine signaling circuit. Cancer Res 2014, 74, 4196-4207, doi:10.1158/0008-5472.Can-14-0582.
- Meier, B.; De Vries, J.; Ziouta, Y.; Basu, A.; Iben, S.; Vander Beken, S.; Hainzl, H.; Sante, L.; Wlaschek, M.; Ganss, C.; et al.et al. Progressive decrease in number, differentiation potential and accumulation of DNA damage of ABCB5+ mesenchymal stem cells in the skin during aging. Exp. Dermatol. 2012, 21, e11, doi: 10.1111/j.1600-0625.2011.01428.x.
- Herold, D.; de Vries, J.C.; Meier, B.; Vander Beken, S.; Jiang, D.; Frank, N.Y.; Kluth, A.; Ganss, C.; Frank, M.H.; Scharffetter-Kochanek, K.; et al. Osteopontin as a potential regulator of dermal ABCB5+ MSC maintenance. Exp. Dermatol. 2017, 26, E13, doi: 10.1111/exd.13280.
- Banerjee, P.; Heit, Y.; Kluth, A.; Ganss, C.; Saab, K.R.; Scharffetter-Kochanek, K.; Murphy, G.F.; Orgill, D.; Frank, M.H.; Frank, N.Y. ABCB5 Promotes Cutaneous Wound Healing through Regulation of a Pro-angiogenic pAKT/HIF1A/VEGF Signaling Cascade. Poster presented at the AAP/ASCI/APSA Joint Meeting, Chicago, Illinois. Available online: https://the-asci.org/wp-content/uploads/2017/04/2017-Joint-Meeting-program.pdf (accessed on 07 Oct 2022)
- García-Bernal, D.; García-Arranz, M.; Yáñez, R.M.; Hervás-Salcedo, R.; Cortés, A.; Fernández-García, M.; Hernando-Rodríguez, M.; Quintana-Bustamante, Ó.; Bueren, J.A.; García-Olmo, D.; et al.et al. The Current Status of Mesenchymal Stromal Cells: Controversies, Unresolved Issues and Some Promising Solutions to Improve Their Therapeutic Efficacy. Front. Cell Dev. Biol. 2021, 9, 650664, doi: 10.3389/fcell.2021.650664.
- Li, C.; Zhao, H.; Cheng, L.; Wang, B.; Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell Biosci. 2021, 11, 187, doi:10.1186/s13578-021-00698-y.
- Singh, K.; Maity, P.; Koroma, A.K.; Basu, A.; Pandey, R.K.; Beken, S.V.; Haas, P.; Krug, L.; Hainzl, A.; Sindrilaru, A.; et al.et al. Angiogenin Released from ABCB5(+) Stromal Precursors Improves Healing of Diabetic Wounds by Promoting Angiogenesis. J. Invest. Dermatol. 2021, 142, 1725-1736, doi: 10.1016/j.jid.2021.10.026.
- Ballikaya, S.; Sadeghi, S.; Niebergall-Roth, E.; Nimtz, L.; Frindert, J.; Norrick, A.; Stemler, N.; Bauer, N.; Rosche, Y.; Kratzenberg, V.; et al.et al. Process data of allogeneic ex vivo-expanded ABCB5(+) mesenchymal stromal cells for human use: off-the-shelf GMP-manufactured donor-independent ATMP. Stem Cell Res. Ther. 2020, 11, 482, doi: 10.1186/s13287-020-01987-y.
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringdén, O.; HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003, 31, 890-896, doi: 10.1016/s0301-472x(03)00110-3.
- Najar, M.; Raicevic, G.; Fayyad-Kazan, H.; De Bruyn, C.; Bron, D.; Toungouz, M.; Lagneaux, L.; Immune-related antigens, surface molecules and regulatory factors in human-derived mesenchymal stromal cells: the expression and impact of inflammatory priming. Stem Cell Rev. Rep. 2012, 8, 1188-1198, doi: 10.1007/s12015-012-9408-1.
- Lee, H.J.; Kang, K.S.; Kang, S.Y.; Kim, H.S.; Park, S.J.; Lee, S.Y.; Kim, K.D.; Lee, H.C.; Park, J.K.; Paik, W.Y.; et al.et al. Immunologic properties of differentiated and undifferentiated mesenchymal stem cells derived from umbilical cord blood. J. Vet. Sci. 2016, 17, 289-297, doi: 10.4142/jvs.2016.17.3.289.
- Zhang, J.; Huang, X.; Wang, H.; Liu, X.; Zhang, T.; Wang, Y.; Hu, D.; The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res. Ther. 2015, 6, 234, doi: 10.1186/s13287-015-0240-9.
- Ankrum, J.A.; Ong, J.F.; Karp, J.M.; Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252-260, doi: 10.1038/nbt.2816.
- Consentius, C.; Reinke, P.; Volk, H.D.; Immunogenicity of allogeneic mesenchymal stromal cells: what has been seen in vitro and in vivo?. Regen. Med. 2015, 10, 305-315, doi: 10.2217/rme.15.14.
- Berglund, A.K.; Fortier, L.A.; Antczak, D.F.; Schnabel, L.V.; Immunoprivileged no more: measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 288, doi: 10.1186/s13287-017-0742-8.
- Sanabria-de la Torre, R.; Quiñones-Vico, M.I.; Fernández-González, A.; Sánchez-Díaz, M.; Montero-Vílchez, T.; Sierra-Sánchez, Á.; Arias-Santiago, S.; Alloreactive Immune Response Associated to Human Mesenchymal Stromal Cells Treatment: A Systematic Review. J. Clin. Med. 2021, 10, 2991, doi: 10.3390/jcm10132991.
- Colbath, A.C.; Dow, S.W.; Phillips, J.N.; McIlwraith, C.W.; Goodrich, L.R.; Autologous and Allogeneic Equine Mesenchymal Stem Cells Exhibit Equivalent Immunomodulatory Properties In Vitro. Stem Cells Dev. 2017, 26, 503-511, doi: 10.1089/scd.2016.0266.
- Berner, A.; Reichert, J.C.; Woodruff, M.A.; Saifzadeh, S.; Morris, A.J.; Epari, D.R.; Nerlich, M.; Schuetz, M.A.; Hutmacher, D.W.; Autologous vs. allogenic mesenchymal progenitor cells for the reconstruction of critical sized segmental tibial bone defects in aged sheep. Acta Biomater. 2013, 9, 7874-7884, doi: 10.1016/j.actbio.2013.04.035.
- Wu, J.; Wang, Q.; Fu, X.; Wu, X.; Gu, C.; Bi, J.; Xie, F.; Kang, N.; Liu, X.; Yan, L.; et al.et al. Influence of Immunogenicity of Allogeneic Bone Marrow Mesenchymal Stem Cells on Bone Tissue Engineering. Cell Transplant. 2016, 25, 229-242, doi: 10.3727/096368915x687967.
- Liew, A.; Baustian, C.; Thomas, D.; Vaughan, E.; Sanz-Nogués, C.; Creane, M.; Chen, X.; Alagesan, S.; Owens, P.; Horan, J.; et al.et al. Allogeneic Mesenchymal Stromal Cells (MSCs) are of Comparable Efficacy to Syngeneic MSCs for Therapeutic Revascularization in C57BKSdb/db Mice Despite the Induction of Alloantibody. Cell Transplant. 2018, 27, 1210-1221, doi: 10.1177/0963689718784862.
- Pan, Q.; Li, Y.; Li, Y.; Wang, H.; Kong, L.; Yang, Z.; Zhang, X.; Bai, S.; Zong, Z.; Chen, G.; et al.et al. Local administration of allogeneic or autologous bone marrow-derived mesenchymal stromal cells enhances bone formation similarly in distraction osteogenesis. Cytotherapy 2021, 23, 590-598, doi: 10.1016/j.jcyt.2020.12.005.
- Hare, J.M.; Fishman, J.E.; Gerstenblith, G.; DiFede Velazquez, D.L.; Zambrano, J.P.; Suncion, V.Y.; Tracy, M.; Ghersin, E.; Johnston, P.V.; Brinker, J.A.; et al.et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 2012, 308, 2369-2379, doi: 10.1001/jama.2012.25321.
- Hare, J.M.; DiFede, D.L.; Rieger, A.C.; Florea, V.; Landin, A.M.; El-Khorazaty, J.; Khan, A.; Mushtaq, M.; Lowery, M.H.; Byrnes, J.J.; et al.et al. Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J. Am. Coll. Cardiol. 2017, 69, 526-537, doi: 10.1016/j.jacc.2016.11.009.
- Kerstan, A.; Dieter, K.; Niebergall-Roth, E.; Dachtler, A.-K.; Kraft, K.; Stücker, M.; Daeschlein, G.; Jünger, M.; Görge, T.; Meyer-Pannwitt, U.; et al.et al. Allogeneic ABCB5(+) mesenchymal stem cells for treatment-refractory chronic venous ulcers: a phase I/IIa clinical trial. JID Innovations 2022, 2, 100067, doi: 10.1016/j.xjidi.2021.100067.
- Girousse, A.; Mathieu, M.; Sastourné-Arrey, Q.; Monferran, S.; Casteilla, L.; Sengenès, C.; Endogenous Mobilization of Mesenchymal Stromal Cells: A Pathway for Interorgan Communication?. Front. Cell Dev. Biol. 2021, 8, 598520, doi: 10.3389/fcell.2020.598520.
- Ullah, M.; Liu, D.D.; Thakor, A.S.; Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience 2019, 15, 421-438, doi: 10.1016/j.isci.2019.05.004.
- Nitzsche, F.; Müller, C.; Lukomska, B.; Jolkkonen, J.; Deten, A.; Boltze, J.; Concise Review: MSC Adhesion Cascade-Insights into Homing and Transendothelial Migration. Stem Cells 2017, 35, 1446-1460, doi: 10.1002/stem.2614.
- Sindrilaru, A.; Peters, T.; Wieschalka, S.; Baican, C.; Baican, A.; Peter, H.; Hainzl, A.; Schatz, S.; Qi, Y.; Schlecht, A.; et al.et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Invest. 2011, 121, 985-997, doi: 10.1172/jci44490.
- Hocking, A.M.; The Role of Chemokines in Mesenchymal Stem Cell Homing to Wounds. Adv. Wound Care 2915, 4, 623-630, doi: 10.1089/wound.2014.0579.
- Sackstein, R.; The lymphocyte homing receptors: gatekeepers of the multistep paradigm. Curr. Opin. Hematol. 2005, 12, 444-450, doi: 10.1097/01.moh.0000177827.78280.79.
- Sackstein, R.; Schatton, T.; Barthel, S.R.; T-lymphocyte homing: an underappreciated yet critical hurdle for successful cancer immunotherapy. Lab. Invest. 2017, 97, 669-697, doi: 10.1038/labinvest.2017.25.
- Riedl, J.; Pickett-Leonard, M.; Eide, C.; Kluth, M.A.; Ganss, C.; Frank, N.Y.; Frank, M.H.; Ebens, C.L.; Tolar, J.; ABCB5+ dermal mesenchymal stromal cells with favorable skin homing and local immunomodulation for recessive dystrophic epidermolysis bullosa treatment. Stem Cells 2021, 39, 897-903, doi: 10.1002/stem.3356.
- Mace, K.A.; Hansen, S.L.; Myers, C.; Young, D.M.; Boudreau, N.; HOXA3 induces cell migration in endothelial and epithelial cells promoting angiogenesis and wound repair. J. Cell Sci. 2005, 118, 2567-2577, doi: 10.1242/jcs.02399.
- Mace, K.A.; Restivo, T.E.; Rinn, J.L.; Paquet, A.C.; Chang, H.Y.; Young, D.M.; Boudreau, N.J.; HOXA3 modulates injury-induced mobilization and recruitment of bone marrow-derived cells. Stem Cells 2009, 27, 1654-1665, doi: 10.1002/stem.90.
- Costa, L.A.; Eiro, N.; Fraile, M.; Gonzalez, L.O.; Saá, J.; Garcia-Portabella, P.; Vega, B.; Schneider, J.; Vizoso, F.J.; Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: implications for further clinical uses. Cell. Mol. Life Sci. 2021, 78, 447-467, doi: 10.1007/s00018-020-03600-0.
- Olmedo-Moreno, L.; Aguilera, Y.; Baliña-Sánchez, C.; Martín-Montalvo, A.; Capilla-González, V.; Heterogeneity of In Vitro Expanded Mesenchymal Stromal Cells and Strategies to Improve Their Therapeutic Actions. Pharmaceutics 2022, 14, 1112, doi: 10.3390/pharmaceutics14051112.
- Levy, O.; Kuai, R.; Siren, E.M.J.; Bhere, D.; Milton, Y.; Nissar, N.; De Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; et al.et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020, 6, eaba6884, doi:10.1126/sciadv.aba6884.
- Srinivasan, A.; Sathiyanathan, P.; Yin, L.; Liu, T.M.; Lam, A.; Ravikumar, M.; Smith, R.A.A.; Loh, H.P.; Zhang, Y.; Ling, L.; et al.et al. Strategies to enhance immunomodulatory properties and reduce heterogeneity in mesenchymal stromal cells during ex vivo expansion. Cytotherapy 2022, 24, 456-472, doi: 10.1016/j.jcyt.2021.11.009.
- European Medicines Agency Committee for Medicinal Products for Human use (CHMP). Guideline on human cell-based medicinal products (EMEA/CHMP/410869/2006). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-human-cell-based-medicinal-products_en.pdf (accessed on 07 Oct 2022).
- U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for Industry: Potency Tests for Cellular and Gene Therapy Products. Available online: https://www.fda.gov/media/79856/download (accessed on 07 Oct 2022).
- Hematti, P.; Characterization of mesenchymal stromal cells: potency assay development. Transfusion 2016, 56, 32s-35s, doi: 10.1111/trf.13569.
- Translational development of ABCB5+ dermal mesenchymal stem cells for therapeutic induction of angiogenesis in non-healing diabetic foot ulcers.; Kerstan, A.; Dieter, K.; Niebergall-Roth, E.; Klingele, S.; Jünger, M.; Hasslacher, C.; Daeschlein, G.; Stemler, L.; Meyer-Pannwitt, U.; Schubert, K.; et al.. Stem Cell Res. Ther. 2022, 13, 455, doi: 10.1186/s13287-022-03156-9.
- Neri, S.; Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int. J. Mol. Sci. 2019, 20, 2406, doi: 10.3390/ijms20102406.
- Tappenbeck, N.; Schröder, H.M.; Niebergall-Roth, E.; Hassinger, F.; Dehio, U.; Dieter, K.; Kraft, K.; Kerstan, A.; Esterlechner, J.; Frank, N.Y.; et al.et al. In vivo safety profile and biodistribution of GMP-manufactured human skin-derived ABCB5-positive mesenchymal stromal cells for use in clinical trials. Cytotherapy 2019, 21, 546-560, doi: 10.1016/j.jcyt.2018.12.005.
- Kiritsi, D.; Dieter, K.; Niebergall-Roth, E.; Fluhr, S.; Daniele, C.; Esterlechner, J.; Sadeghi, S.; Ballikaya, S.; Erdinger, L.; Schauer, F.; et al.et al. Clinical trial of ABCB5+ mesenchymal stem cells for recessive dystrophic epidermolysis bullosa.. JCI Insight 2021, 6, e151922, doi: 10.1172/jci.insight.151922.
- Hartwig, V.; Dewidar, B.; Lin, T.; Dropmann, A.; Ganss, C.; Kluth, M.A.; Tappenbeck, N.; Tietze, L.; Christ, B.; Frank, M.; et al.et al. Human skin-derived ABCB5(+) stem cell injection improves liver disease parameters in Mdr2KO mice. Arch. Toxicol. 2019, 93, 2645-2660, doi: 10.1007/s00204-019-02533-3.
- Tietze, L.; Winkler, S.; Hempel, M.; Kluth, M.A.; Tappenbeck, N.; Ganss, C.; Dooley, S.; Christ, B.; Assessment of the hepatocytic differentiation ability of human skin-derived ABCB5(+) stem cells. Exp. Cell Res. 2018, 369, 335-347, doi: 10.1016/j.yexcr.2018.05.040.
More
