Electrospun polymer nanofibers constitute one of the most important nanomaterials with diverse applications. Nanofibers are classified as fibers with a ratio of length to thickness in the order of one thousand, or nanomaterials that have at least one dimension of 100 nm or less. A nanofiber with a diameter of ∼100 nm can have a specific surface area up to 1000 m2/g. Nanofibers can be produced by selecting the proper combination of polymers and additives, and using appropriate production techniques based on several essential characteristics that impact criteria of the intended particular application area. Electrospinning of polymer nanofibers is a widely used for investigation of their properties for uses in quite diverse applications. Attractive properties of electrospun nanofibers include the extremely high specific surface area, high porosity (typically 90%), light weight, controllable pore size, flexibility in surface functionalities, large permeability, excellent mechanical properties, high aspect ratio, and length up to many centimeters. Due to their exceptional characteristics, electrospun polymer nanofibers are used in many applications, which include biomedical technology, such as tissue engineering, wound healing and dressing, and drug delivery systems. In addition they have diverse uses in sensors and biosensors applications, air filtration, defense applications, energy devices and protective textiles.
- polymer nanofibers
- electrospinning
- polymer processing
- mechanical properties
- biomedical application
- energy storage separation
1. Polymers Used in Electrospinning
1.1. Natural and Synthetic Polymers
1.2. Composite Polymers/Copolymers
2. Electrospinning Process Parameters
-
Parameters related to the solution, such as polymer molecular weight, polymer concentration, surface tension, conductivity, solvent volatility, and viscosity.
-
Parameters related to processing such field strength, flow rate, tip-to-collector separation, applied voltage, placement and design of the needle tip, composition and geometry of the collector, and take-up velocity of the collector.
-
Properties related to environmental factors including temperature, humidity, and pressure.
2.1. Parameters Related to the Polymer Solution
2.1.1. Concentration of the Polymer
2.1.2. The Solvent
2.1.3. Electrical Conductivity
2.1.4. Viscosity
2.1.5. Molecular Weight
2.2. Parameters Related Electrospinning Equipment
2.2.1. Applied Voltage
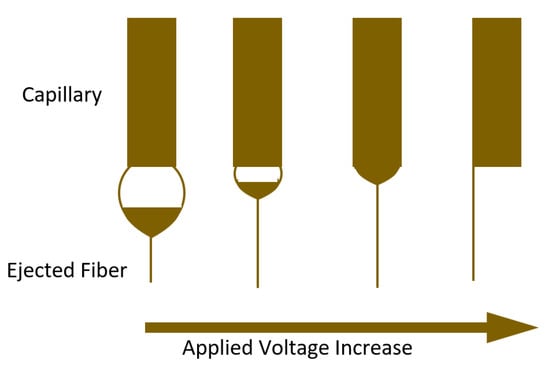
2.2.2. Feed Rate
2.2.3. Distance between Tip and Collector
2.3. Parameters Related to Environment
3. Properties of Polymer Nanofibers
3.1. Mechanical Properties
-
A manipulation system that precisely isolates, aligns, and grasps a single nanofiber on a frame without slipping or damaging.
-
A proper monitoring system to verify that nanofibers are not harmed by characterization tools such as scanning electron microscopes or transmission electron microscopes.
-
A sensitive force transducer having a range of nano- to micro-Newton range (n/μN range) resolution that can measure applied force in the n/μN range.
-
An actuator that is capable to load nanofibers until fracture, with high resolution (load unit: μN).
3.2. Chemical Properties
Fourier-transform infrared spectroscopy, nuclear magnetic resonance, circular dichroism, differential scanning calorimetry, X-ray scattering, and X-ray diffraction are commonly used to characterize the chemical composition of nanofibers. The vibrational spectroscopic technique is used for molecule structure analysis. This approach assists in determining chemical reactions between ingredients of polymers in case of polymer blends [11][23]. Raman spectroscopy is utilized to examine the structural characteristics of carbonaceous polymers. This was used by Sadrjahani et al. to assess the molecular orientation of electrospun polyacrylonitrile nanofibers [28][145]. A chain alignment parameter of 0.25 was calculated for nanofibers which accumulated at a 59.5 m/min take-up velocity. Surface chemical characteristics of polymer nanofibers can be analyzed by making use of their hydrophilicity. The latter is calculated by water contact angle, and it is examined by attenuated total reflectance Fourier transform infrared and X-ray photoelectron spectroscopy. Element detection by X-ray photoelectron spectroscopy is possible up to depth of 100 Å [29][146]. This technique is used to check the shell within core-shell structure of electrospun nanofibers, not to form a blend or react chemically with core [30][147]. Supermolecular structures, which are referred to as macromolecular configurations in polymer nanofibers, can be examined by small angle X-ray scattering, wide angle X-ray diffraction and differential scanning calorimetry [31][148]. The crystalline phase and crystal type are identified by wide angle X-ray diffraction, while small angle X-ray scattering is used to examine the lamellar structure of semicrystalline polymers. Other equipment is not used commonly due to complications in interpreting its pattern.3.3. Thermal Properties
Thermal characterizations of electrospun nanofibers, such as melting and crystallization processes, can be determined by differential scanning calorimetry. To carry out this procedure, electrospun mats weighing around 10 mg are placed in a sealed aluminum pan. To preserve ambient pressure and promote evaporation of leftover solvents, pan covers are equipped with holes. Then, the specimens’ temperature is raised from 30 °C to 300 °C while maintaining a heating rate of usually 10 °C/min and a steady flow of dry nitrogen. The following equation determines the percent crystallinity (χc) [32][149]: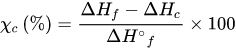 χc(%)=ΔHf−ΔHcΔH∘f×100
χc(%)=ΔHf−ΔHcΔH∘f×100 3.4. Electrical Properties
To analyze the electrical properties of nonwoven mats and single fibers, electrodes are pre-patterned on a substratum or vaporized on top of electrospun polymer materials [36][153]. To measure electrical conductivity readings from current voltage curves, generally four-point probe [37][154] or two-point probe [38][155] measurements are utilized. When using a four-point probe, a material of unknown resistance is contacted by four evenly spaced probes. Measurements based on this technique assume a thin film instead of a porous fiber network. As a result, measured conductivity measurements can be lower than the actual measurement for bulk films. Another source of uncertainty for electrical conductivity is the pins’ depth (height) of penetration into fiber mats [39][156]. Penetration of pins into a polymer fiber mat could be the cause of uncertainty in determining the electrical conductivity. Utilizing interdigitated electrodes to measure electrospun nanofibers electrical conductivity is an alternative approach [40][158]. According to Zhang and Rutledge, electrical contact can be established by applying hot pressure of nanofibers on electrodes [41][159]. Contact resistance is calculated by determining overall fiber resistance on interdigitated electrodes having varying finger spacing and with the help of extrapolating resistance value at zero spacing. It is observed that conductivities of fibers increased exponentially by weight fraction of doped polyaniline in fibers; the value for completely doped electrospun fiber was 50 ± 30 S/cm and increased to 130 ± 40 S/cm on further solid state drawing [41][159]. Electrospinning of electrically conductive polymers primarily focuses on polyaniline and its blends. Extremely conductive electrospun polyaniline fiber doped in sulfuric acid is produced by a blend of polyaniline and various conventional polymers such as polystyrene, polyacrylonitrile, polyethylene oxide, and so onetc. [42][160].3.5. Optical Properties
Nagata et al. conducted a lot of optical characterization techniques utilizing a luminescence spectrofluorometer and ultraviolet visible spectrophotometers to study optical characteristics of poly (2-methoxy- 5-(2-ethylhexyloxy)-1,4-phenylenevinylene) electrospun nanofibers [43][161]. A remarkable red shift was detected at all concentrations of MEH-PPV electrospun nanofibers in comparison to thin film. Photoluminescence readings validated the red shift by raising polymer concentration. Babel et al. observed the electrospun nanofiber’s optical properties of conjugated polymer blends utilizing ultraviolet visible spectrophotometers, near-infrared spectroscopy, and photoluminescence spectroscopy [44][162]. Their finding shows that conjugated polymers’ binary blend has an adjustable composition based on optical characteristics that can be exploited in field-effect transistors. Balderas et al. observed analogous absorption of red shift electrospun fibers generated from a blend of poly(9-vinylcarbazole) and MEH-PPV [45][163].4. Applications
4.1. Biomedical Applications
One of the most significant applications of polymer nanofibers is in the biomedical sector, particularly in the domains of medication delivery and tissue engineering. Given that biological molecules and nanoscale fibers have similar sizes, the latter are poised to perform well in simulating biological environments and natural extracellular matrices. Nanofibrous meshes exhibit enhanced biological activities, such as increased cell adhesion, differentiation, and proliferation, due to high porosity, large surface area to volume ratio, and interconnectivity of porous matrices comparable to macromolecular ones. Additionally, it is also conceivable for biological molecules to load for nutrients and wastes to exchange through pores [10][46]. The two main research directions in this field are explored below.One of the most significant applications of polymer nanofibers is in the biomedical sector, particularly in the domains of medication delivery and tissue engineering. Given
that biological molecules and nanoscale fibers have similar sizes, the latter are poised to perform well in simulating biological environments and natural extracellular matrices.
Nanofibrous meshes exhibit enhanced biological activities, such as increased cell adhesion, differentiation, and proliferation, due to high porosity, large surface area to volume ratio, and interconnectivity of porous matrices comparable to macromolecular ones. Additionally, it is also conceivable for biological molecules to load for nutrients and wastes to exchange through pores [22,192]. The two main research directions in this field are explored below.
4.1.1. Tissue Engineering
Electrospun polymer nanofibers utilized as scaffolds in tissue engineering have attracted much attention. Due to comparable fibrous structure of nanofibrous made of biodegradable polymers with natural extracellular matrices, they operate so as to support cell proliferation, adhesion, and differentiation. As such, they possess great potential as scaffolds for tissue regeneration [47][48][179,193]. Collagen, keratin, elastin fibers, and so onetc. obtained from extracellular matrix are the most often employed materials in this endeavor because they are inherently fibrous in nature and easily transformed into fibrous scaffolds.
4.1.23. Wound Healing and Dressing
In wound rehabilitation pursuit, the large porosity of electrospun fibers may provide additional structural space for accommodation of transplanted cells, promote cell migration and proliferation, and increase oxygen exchange and waste outflow. The tiny pore size of nanofibrous scaffolds can prevent dehydration and wound infection throughout the healing process. Additionally, tunable mechanical properties of electrospun nanofibers can maintain mechanical consistency between tissue engineering grafts and parent tissue and prevent the wound from wrinkling or shrinkage during implantation. A variety of synthetic biodegradable polymers such as polyglycolic acid, polylactic acid, polycaprolactone, and copolymers are generally used for skin tissue engineering because of their advantageous mechanical and biodegradable characteristics.
4.1.34. Drug Delivery Systems
Various drug delivery systems such as polymer micelles, liposomes, and nanofibers are studied to diminish the toxicity of dosage and increase therapeutic efficacy [49][50][51][52][198–201]. There is a potential for electrospun nanofibers to provide significant benefit due to flexibility in selection of materials and medications, encapsulation efficiency, and delivery of therapeutic agents, among others, which makes them appealing candidates in drug delivery, particularly for topical chemotherapy after surgery and in wound casing materials [53][37]. Electrospun nanofibers are utilized in precise and localized drug delivery systems thanks to their main advantages of large surface to volume ratios and well-interlinked, open porous structure. Numerous attempts have been undertaken to integrate bioactive compounds after electrospinning them, either chemically or physically, into the scaffolds. Techniques such as blending, co-axial electrospinning, and surface modification are utilized to load drugs into nanofibers.
4.2. Sensors and Biosensors
Significant advancements have been achieved in the manufacturing of extremely biological and chemical sensitive sensors in response to increasing demands for high-precision reliable detections in different and evolving applications in medicine and sophisticated manufacturing for targeted industries. Electrospun polymer nanofibers provide a fertile source of utilization in sensing applications. An optical sensor that was developed by the electrospinning of fluorescent polymer nanofibers showed three-time improved sensitivity magnitude as compared to film sensors for detection of mercury ions and nitro and ferric compounds. Conductive electrospun polymer nanofibers such as polyaniline nanowires are strong candidates for sensing applications due to their outstanding electrical characteristics [54][55][56][203–205].
4.3.
4.3.
Air Filtration
Various studies have suggested that electrospun nanofibers have the ability to capture such volatile organic compounds in air. Electrospun polymer nanofiber membranes have shown faster adsorption and desorption of volatile organic compounds compared to conventional activated carbon. The performance of filter membranes is significantly influenced by the structural properties of electrospun fibrous membranes. Fiber diameter and distribution, pore size distribution, surface area, basis, and density constitute the determining factors for filtering process effectiveness. Polymer nanofibers with smaller diameter will have a more accessible surface area, which will reduce pressure drop. Therefore, selecting an optimal electrospun nanofiber diameter is essential to maximizing filtration performance.
4.4. Defense Applications
Polymer nanofibers are considered as exceptional membrane materials for the defense industry in smart textiles for the detection of biological and chemical warfare agents with high sensitivity. Their high sensitivity to biological and chemical pollutants at concentrations of parts per billion make them attractive candidates for sensing interfaces for warfare agents. Studies were conducted to incorporate nanoparticles into an electrospun nanofiber to enhance their properties for effective utilization in smart textiles. In electrospinning polymers such as polyamide, polypropylene, polyvinyl alcohol, and so onetc., their composites are utilized.
4.5.
4.5.
Energy Devices
It was reported that nanofibers perform better than typical materials in devices for energy storage, harvesting, and conversion, offering good alternative materials for use in energy devices such as lithium-ion batteries, nanogenerators, and solar cells [57][6]. Nanofibers used in solar cells have shown high photoelectric conversion efficiency due to separation, efficient charge transmission, and high light absorption mainly due to a large specific surface area and high porosity. The large ratio of surface area to volume in nanofibers enhances formation of the nonwoven structure, which improves conductivity and gives rise to possible utilization of NF in batteries and fuel cells as a separation medium. Nanofiber-based electrodes in solar cells have shown high cycling stability and specific capacity.
