Migraine is a chronic neurological disorder that affects approximately 12% of the population. The cause of migraine headaches is not yet known, however, when the trigeminal system is activated, neuropeptides such as calcitonin gene-related peptide (CGRP) and substance P (SP) are released, which cause neurogenic inflammation and sensitization. Advances in the understanding of migraine pathophysiology have identified new potential pharmacological targets. Transient receptor potential (TRP) channels have been the focus of attention in the pathophysiology of various pain disorders, including primary headaches. Genetic and pharmacological data suggest the role of TRP channels in pain sensation and the activation and sensitization of dural afferents. TRP channels are widely expressed in the trigeminal system and brain regions which are associated with the pathophysiology of migraine and furthermore, co-localize several neuropeptides that are implicated in the development of migraine attacks. Moreover, there are several migraine trigger agents known to activate TRP channels. Based on these, TRP channels have an essential role in migraine pain and associated symptoms, such as hyperalgesia and allodynia. Mammalian TRP channels are divided into seven subfamilies based on their homology of amino acid sequences: canonical or classic (TRPC), vanilloid (TRPV), melastatin (TRPM), nonmechanoreceptor potential C (NOMP-like, TRPN1) polycystin (TRPP), mucolipin (TRPML), and ankyrin (TRPA).
1. Characterization of Transient Receptor Potential Vanilloid PV1 and Role in Pain and Headaches
One of the first
transient receptor potential (TRP
) channels to be investigated was
transient receptor potential vanilloid 1 (TRPV1)TRPV1, which is a nonselective cation channel responsive to high temperature (>43 °C) and capsaicin (the main pungent ingredient in “hot” chili peppers)
[1][63], which have been shown to activate sensory nerves and induce neurogenic inflammation (NI)
[2][64]. TRPV1 is also sensitive to endocannabinoids, endovanilloids, nerve-growth factor (NGF), and prostaglandins (PGs), which may be relevant for migraine
[3][65]. The hydrogen ion, acid, or low pH also can activate the TRPV1 channel
[4][66] (
Figure 1 and
Figure 2).
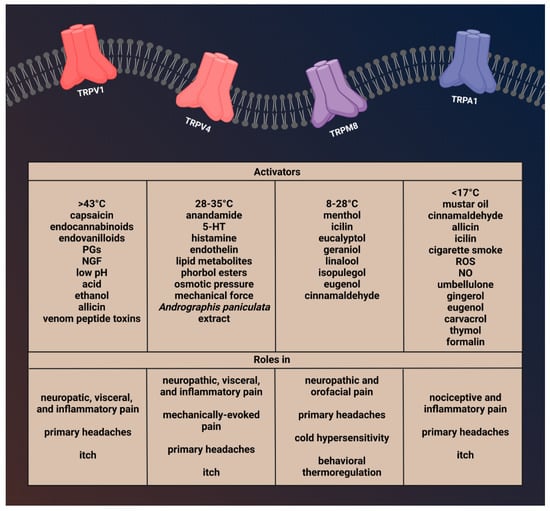
Figure 1.
Activators and function of transient receptor potential channels involved in migraine.
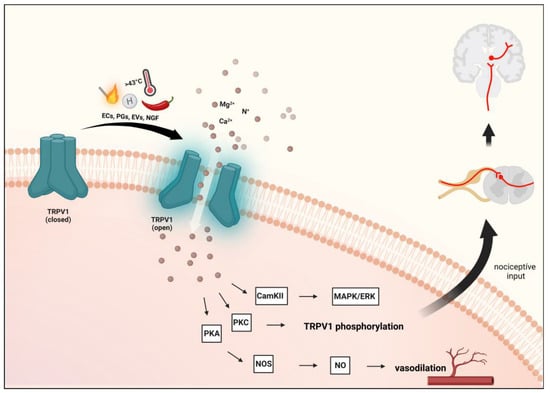
Figure 2. Transient receptor potential vanilloid 1 receptor activation. Activation of TRPV1 and the resulting influx of cations can further activate voltage-gated ion channels to generate action potentials to be required for pain or itch signaling. Several inflammatory mediators lower the activation threshold of TRPV1 via phosphorylation mainly through the activation of the cAMP-dependent protein kinase A (PKA) pathway. Furthermore, protein kinase C (PKC)-dependent cascade is also involved. TRPV1: transient receptor potential vanilloid 1 receptor, ECs: endocannabinoids, EVs: endovanilloids, PGs: prostaglandins, NGF: nerve growth factor.
Several studies have shown that approximately 40–50% of trigeminal sensory neurons express TRPV1
[5][67]. Furthermore, it is expressed in small amounts in the hypothalamus, hippocampus, entorhinal cortex, raphe nucleus, and the periaqueductal gray matter (PAG)
[6][7][8][9][68,69,70,71]. TRPV1 receptor is present in small and medium-diameter neurons of the dorsal ganglion (DRG) and trigeminal ganglia (TG), colocalized with
calcitonin gene-related peptide (CGRP
) and substance P (SP) and SP in the latter
[1][63]. In addition, TRPV1 and NMDAR are coexpressed in the TG
[10][72]. However, TRPV1 has also been described in brain areas that are not associated with pain or heat sensations, such as the ventral tegmental area or the striatum
[11][12][73,74].
Upon activation of TRPV1, CGRP and SP are released, causing vasodilation and triggering NI in the meninges
[13][14][75,76]. Furthermore, in sensory neurons, activation of TRPV1 by NO leads to peripheral sensitization and nociception
[15][77].
After tissue damage, endogenously released inflammatory mediators such as bradykinin, serotonin (5-HT), PGs, or histamine can influence TRPV1 activity, mainly indirectly through the stimulation of their receptors and the generation of second messengers
[16][17][78,79]. TRPV1 is a molecular component of pain sensation and modulation
[18][80]. Activation of TRPV1 and the resulting influx of cations can further activate voltage-gated ion channels to generate action potentials required for pain or itch signaling
[5][67]. The sensitization and endogenous regulatory pathways of TRPV1 can exert their effects through the phosphorylation sites of protein kinases C (PKC) and A (PKA) and Ca
2+/calmodulin-dependent kinase II (CAMKII)
[19][20][30,81]. Prolonged or repeated activation of TRPV1 prompts a desensitization or inhibition process
[17][79], thereby losing the sensitivity to capsaicin and other chemical agonists, further reducing the sensitivity to heat
[21][82].
2. Brief Description of Transient Receptor Potential Vanilloid PV4 and Its Role in Pain and Headache
Receptor Potential Vanilloid 4 (TRPV4
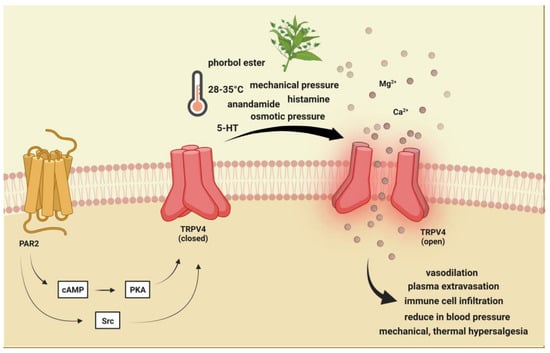
) is a polymodal cation channel activated by moderate heat (>24 °C to 27−35 °C), low pH, endocannabinoids, lipid metabolites, osmotic pressure, and phorbol ester and plant-derived compounds
[22][23][24][108,109,110]. It plays a crucial role in mechanical-, thermal-, and chemical-induced pain sensitivity
[25][111]. TRPV4 is also involved in the regulation of vascular tone and acute inflammatory signaling
[26][27][112,113] and functions as part of the mechanosensory complex. Based on these, the functions and trigeminal localization of TRPV4 may fit some aspects of migraine, such as the characteristic throbbing pain that is aggravated by routine movements, coughing, or sneezing
[28][52]. Another finding supporting the role of TRPV4 in migraine is that solutions applied to the surface of the dura mater that increase or decrease osmolarity can sensitize trigeminal afferents
[29][30][31][114,115,116]. TRPV4-dependent pathways promote plasma extravasation and immune cell infiltration by increasing the release of some neuropeptides, including CGRP and SP, and thus are considered to be potentiators of neurogenic inflammation
[32][117] (
Figure 1 and
Figure 3).
Figure 3. Transient receptor potential vanilloid 4 receptor activation. TRPV4 is activated by moderate heat (>24 °C to 27−35 °C), low pH, endocannabinoids, lipid metabolites, osmotic pressure, and phorbol ester and plant-derived compounds. PAR-2 activation may indirectly sensitize (via PKA, PKC, and PLC) TRPV4, thereby contributing to mechanical allodynia and thermal hyperalgesia. TRPV4: Transient receptor potential vanilloid 4, 5-HT: serotonin.
TRPV4 is widely expressed in various regions in the PNS and CNS, including immune cells, hippocampal neurons, nonpeptidergic, Aβ and Aδ fibers neurons of DRG, and peptidergic C fibers, where it coexpresses with TRPV1
[33][118]. Aside from the neurons TRPV4, is also present in nonmyelinating Schwann cells and satellita glial cells
[34][119]. TRPV4 mRNA is expressed in TG, and in vitro investigations prove functional effects of receptor on trigeminal neurons
[35][120]. Furthermore, TRPV4 shows colocalization with CGRP, SP, and protease-activated receptor 2 (PAR2) in rat sensory neurons
[36][121]. In addition to PAR2, the role of TRPV4 in inflammation has also been associated with histamine and serotonin. Histamine- or serotonin-induced visceral hypersensitivity is significantly reduced when TRPV4 is blocked with siRNA, indicating TRPV4-dependent histamine- or serotonin-mediated response in sensory neurons
[37][122].
3. Brief Description of TRansient Receptor Potential Melastatin 8 PM8 and Its Involvement in Pain and Headache
The T
ransient R
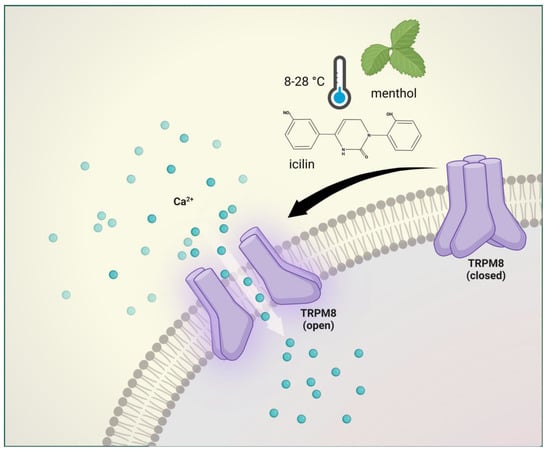
eceptor Potential Melastatin 8 (TRPM8)PM8 is a nonselective cation channel with modest calcium permeability and is activated by cold temperatures (8–28 °C), membrane depolarization, menthol, and icilin
[38][142].
TRPM8 is expressed on C- and Aδ- sensory nerve fibers, as well as DRG and TG neurons
[39][143]. A subset of TRPM8-positive cells may coexpress TRPV1 and/or CGRP
[40][41][42][144,145,146]. Furthermore, it is present in hypothalamic and hindbrain nuclei responsible for autonomic thermoregulation
[43][147]. In addition, TRPM8 is also expressed in macrophages. Activation of TRPM8 on macrophages increases the release of interleukin 10 (IL-10) and decreases the release of tumor necrosis factor (TNF), thereby causing an anti-inflammatory response
[44][148] (
Figure 1 and
Figure 4).
Figure 4. Transient receptor potential melastatin 8 receptor activation. The TRPM8 is activated by cold temperatures (8–28 °C), membrane depolarization, menthol, and icilin. TRPM8 mediates normal thermosensation and has a role in both cooling-mediated analgesia and cold hypersensitivity.
The TRPM8 has been shown to play a major physiological role in inflammation, thermoregulation, itch, and migraine
[45][46][47][48][149,150,151,152]. In addition, TRPM8 mediates normal thermosensation and has a role in both cooling-mediated analgesia and cold hypersensitivity after injury
[41][49][145,153]. TRPM8 has been identified in several genome-wide association studies (GWAS) as one of the migraine susceptibility genes
[50][51][154,155]. There is an association between migraine incidence and single nucleotide polymorphisms located near the TRPM8 coding region, although this seems to be the case mainly for people of Northern European ancestry
[52][156]. It is currently unknown how these genetic variants affect the function or expression of TRPM8 and what is their role in migraine. Furthermore, about 50% of migraine patients have cold allodynia
[53][157], which further strengthens the role of TRPM8 in the disease.
4. Brief Description of Transient Receptor Potential Ankyrin 1 PA1 Channel and Role in Pain and Headaches
T
ransient R
eceptor Potential Ankyrin 1 (TRPA1) PA1 is a nonselective cation channel with an inward depolarizing current due to Na
+ and Ca
2+ ions
[54][168]. TRPA1 channels play a role in the detection of pungent or irritating substances, such as allyl isothiocyanate (mustard oil), allicin, and diallyl disulfide (garlic)
[55][56][169,170]. Moreover, gingerol (ginger), eugenol (cloves), carvacrol (oregano), and thymol (thyme) can also activate this receptor
[57][58][59][171,172,173]. There are conflicting results that mechanical stimuli and noxious cold (<17 °C) also affect TRPA1 function
[60][61][174,175]. In addition, evidence suggests that bradykinin and prostaglandins can indirectly activate TRPA1 by the activation of kinase proteins and second messengers
[62][63][176,177].
It is present in subpopulations of primary sensory neurons of the DRG, TG, and vagal ganglia (VG)
[60][174]. TRPA1 is mainly expressed in unmyelinated C-fibers and thinly myelinated Aδ-fibers
[60][174]. Although TRPA1 is mainly located in nociceptive neurons of the PNS, it is also found at different sites of the CNS, such as in the cortex, caudate nucleus, putamen, globus pallidus, substantia nigra, hippocampus, cerebellum, amygdala, and hypothalamus
[64][50]. In primary sensory neurons, TRPA1 is coexpressed with SP, CGRP, and TRPV1 in primary sensory neurons, and after neuronal activation, the release of these peptides produces neurogenic inflammation and vasodilatation in the dura
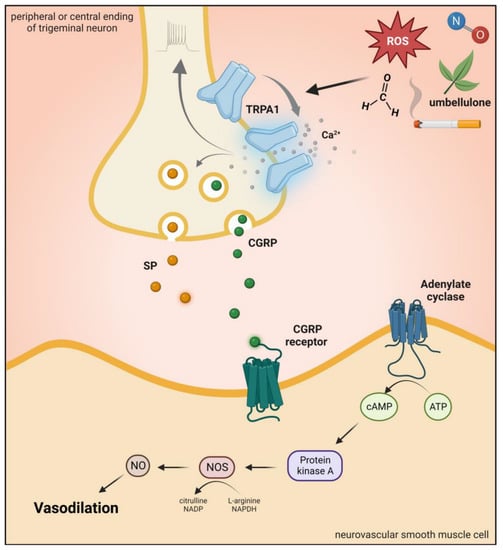
[55][60][65][66][169,174,178,179] (
Figure 1 and
Figure 5).
Figure 56. Transient receptor potential ankyrin 1 receptor activation. Activation of TRPA1 in sensory neurons induces an increase in Ca2+ and leads to the release of the neuropeptide CGRP, SP, and NOS-derived NO, thus mediating vasodilation. TRPA1: transient receptor potential ankyrin 1 receptor, CGRP: calcitonin gene-related peptide, SP: substance P, ROS: reactive oxygen species.