Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by raj nayan sewduth.
Lung cancer is one of the most common cancers worldwide. It consists of two different subtypes: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Despite novel therapeutic options such as immunotherapy, only 20% of lung cancer patients survive the disease after five years. This low survival rate is due to acquired drug resistance and severe off-target effects caused by currently used therapies. Identification and development of novel and targeted therapeutic approaches are urgently required to improve the standard of care for lung cancer patients.
- lung cancer
- drug delivery
- adenovirus
- PROTAC
1. Adenoviral Targeting of Oncogenic Protein Modifications
Adenoviruses, many of which are responsible for respiratory infections in humans, have been genetically engineered to allow the delivery of genetic material to cells. These viruses demonstrate the ability to target a various range of cell types, as it is possible to use different serotypes that have specific affinity to specific cell types. Adenoviruses have a strong affinity for lung epithelial cells, from which most lung cancers are driven. Adenoviral-based therapies are genetic therapies that enable the expression of a protein of interest at a high level without showing any DNA integration or immune response towards the recombinant adenovirus. The efficiency of such therapies has been demonstrated in several genetic diseases (i.e., Zolgensma) and, more recently, by the use of adenoviral-based vaccines against COVID-19 respiratory diseases. Several clinical trials have tested with some degree of success adenovirus-based approaches to impair oncogenesis, based on specific cancer-specific genetic mutations such as the one affecting p53 function,. On the other hand, clinical trials focusing on the immune response, such as anti-dendritic cell or cytokine therapy, were less successful but demonstrated an interesting proof-of-concept approach [3,10][1][2]. The large number of clinical trials using adenoviral-based approaches indicates the important potential of adenoviral-based therapies for lung cancer, especially considering the recent acceptance of the adenovirus-based vaccines in the general population with the COVID-19 vaccines. Further optimizations are still required to reach the full potential of adenoviral-based therapies in cancer patients.
2. PROteolysis TArgeting Chimeras (PROTACs) Based Therapies
Degradation of targeted proteins using proteolysis targeting chimeras (PROTACs) has recently gained a lot of momentum. A PROTAC is a bifunctional molecule that consists of different parts: a ligand that interacts with the protein to be degraded and another ligand that binds to an E3 ubiquitin ligase to promote the degradation of a specific target. The efficacy of PROTACs has been shown to be hard to predict, especially in the context of cancer. A recent article indicated that the design of the PROTACs should be optimized for each type of cancer [24][3], as the level of E3 ligases/degradable targets is different in each. In the case of lung cancer, different approaches were tested, as listed in Figure 1/Table 31 below. Recent clinical trials demonstrate the safety and the retainment of pharmacokinetic and therapeutic efficiency of the PROTACs in the body. The delivery of PROTACs to patients was challenging in the past, as the size of PROTACs was too important, impairing cell cellular membrane penetration. Recent optimizations for the delivery of the molecules include switching from Von Hippel Lindau (VHL) to Cereblon (CRBN)-recruiting/phthalimide-based PROTACs, which show better bioavailability even when delivered orally to patients. VHL is often mutated in Von Hippel–Lindau disease, possesses E3 ubiquitin ligase activity, and acts as an adaptor of the Cullin-2 (CUL2) RING ubiquitination complex. One of the main substrates of VHL is HIF1 (hypoxia-inducible factor 1), whose level is finely regulated through ubiquitination in every cell type. CRBN, on the other hand, is an E3 ubiquitin ligase adaptor of the CUL4A ubiquitination complex with a strong affinity for damaged DNA binding protein 1 (DDB1). Both ubiquitin adaptors are expressed at high levels and present viable options for the design of PROTACs. This shows that while PROTACs’ size can be seen as a problem for delivery, it is still possible to optimize PROTACs by reducing their size or changing their recruiter to maximize their delivery of tumor cells in patients. The PROTACs present the advantage of being able to target a specific oncogenic mutation or a specific genetic interaction between two proteins, only present in tumor cells.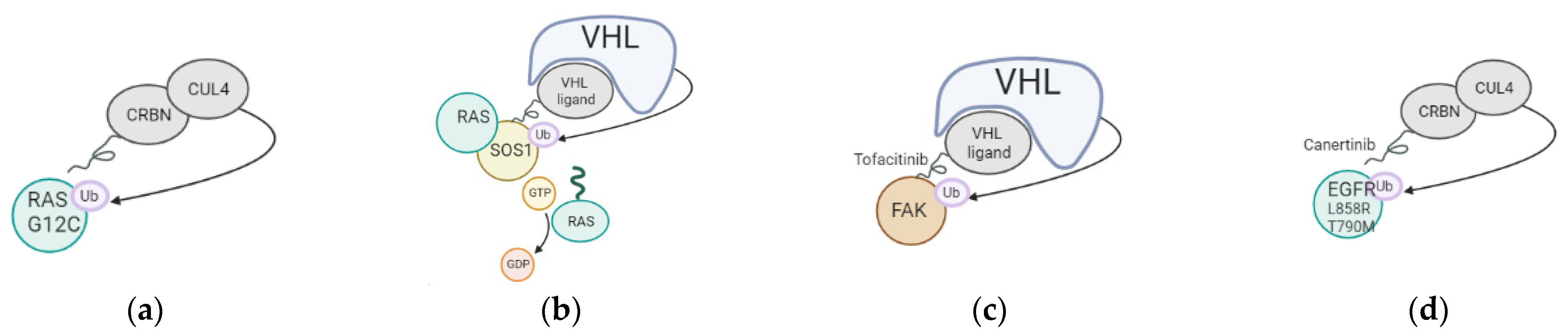
Figure 1. Mechanism of action of PROTACs tested in lung cancer. (a) Targeting of KRAS G12C oncogenic mutation using a Cereblon-based PROTAC; (b) Targeting of SOS1, cofactor of KRAS, using a VHL-based PROTAC; (c) Targeting of FAK using a VHL-based PROTAC; (d) Targeting of EGFR L858R and T790M using a CRBN-based PROTAC.
Table 31.
PROTACs tested in lung cancer with details of degrader and recruited ligase.
3. Lipid Nanoparticle-Based Therapies
Delivery of an active drug or gene therapy to target tumor growth has been successfully achieved through specific structures, called lipid nanoparticles (LNPs), engineered to preserve the active components’ function while reducing side effects. The first attempt at cancer immunotherapies in 1995 was based on mRNA delivery via LNPs, when injection of mRNA encoding carcinoembryonic antigens elicited antigen-specific immune responses in mice. Following the wide acceptance of lipid nanoparticle-based anti-COVID-19 (Coronavirus Disease 19) vaccines [37][10], a number of mRNA-based cancer vaccines are currently undergoing clinical trials based on lipid nanoparticle–mRNA formulations. So far, most clinical trials have focused on delivering classical clinical drugs, such as docetaxel/paclitaxel, or PD1 (programmed cell death 1), in combination with cancer-specific antigens (FixVac-PD1) to increase the immune response [38][11] (Table 42). Recent studies have also targeted kinases or phosphatases involved in the oncogenic signaling cascade. While not all focusing on lung cancer, previous clinical studies on solid/epithelial tumors show that the lipid nanoparticle-based treatments are generally well tolerated with few side effects in patients, at least during the monitoring of the trials. Further investigation is required to assess the long-term effect of lipid nanoparticle delivery, especially on the cardiovascular system. It is also possible to target the particles to specific cell types using specific adjustments to the lipid nanoparticles coating the vesicles. Several examples were described, including targeting specific immune cell populations to impair tumor progression [39][12], tumor cells directly, or tumor associated vessels [40][13]. This targeting involves various modifications, such as magnetoliposomes, that enable specific cell targeting of specific cell types according to their magnetic properties. Targeting the lipid nanoparticles to specific cell types would reduce any side effects to a minimaum, enabling the safest setup for such therapies. As described in Table 42, some clinical trials involving nanoparticles reached Phase III and IV, showing the acceptance of this strategy in the targeting of cancer progression. These phase III and IV trials included different targets, including the PD1 receptor, paclitaxel, or A-type CpG-oligonucleotides specific to cancer cells (Table 42, [41,42,43][14][15][16]).Table 42.
Clinical trials with lipid nanoparticles in solid tumors.
| Study Type | Target | Status | Reference |
|---|---|---|---|
| Phase II | BIND-014 (docetaxel nanoparticles) in lung cancer | Completed | [44][17] |
| Phase II | BIND-014 (docetaxel nanoparticles) in KRAS-mutated lung cancer | Completed | [45][18] |
| Phase II | Carboplatin and paclitaxel albumin-stabilized nanoparticle (ovarian) | Completed | [46][19] |
| Phase II | Paclitaxel albumin-stabilized nanoparticle in lung cancer | Completed | [47][20] |
| Phase I-II | ABI-007 (albumin-stabilised paclitaxel nanoparticle) in lung cancer | Completed | [48][21] |
| Phase II | CRLX101 (cyclodextrin-based polymer with camptothecin) in lung | Completed | [49][22] |
| Phase IV | Paclitaxel liposome in lung cancer | Completed | [41][14] |
| Phase III-IV | A-type CpG-oligonucleotides (CpG-ODN) coupled to peptide derived from Melan-A/MART-1 in melanoma | Completed | [42][15] |
| Phase III | Paclitaxel micelles in lung cancer | In progress | [50][23] |
| Phase I-II | GPX-001 (TUSC2 encapsulate in lipid nanoparticles) in lung cancer | In progress | [51][24] |
| Phase II | LY01610 (irinotecan hydrochloride liposome) in lung cancer | In progress | [52][25] |
| Phase II-III | ONIVYDE (irinotecan liposome) in lung cancer | In progress | [53][26] |
| Phase III | HLX10 (humanized—PD-1 receptor) liposome in lung cancer | In progress | [43][16] |
| Phase I-II | CRLX101 (cyclodextrin polymer with camptothecin) in lung cancer | In progress | [54][27] |
| Phase II | ABI-009 (albumin-bound mTOR inhibitor) in neuroendocrine tumor | Terminated | [55][28] |
References
- To Immunize Patients with Extensive Stage SCLC Combined with Chemo with or without All Trans Retinoic Acid. Available online: https://clinicaltrials.gov/show/NCT00617409 (accessed on 7 December 2022).
- Available online: https://clinicaltrials.gov/show/NCT02140996 (accessed on 7 December 2022).
- Nieto-Jiménez, C.; Morafraile, E.C.; Alonso-Moreno, C.; Ocaña, A. Clinical considerations for the design of PROTACs in cancer. Mol. Cancer 2022, 21, 1–9.
- Zeng, M.; Xiong, Y.; Safaee, N.; Nowak, R.P.; Donovan, K.A.; Yuan, C.J.; Nabet, B.; Gero, T.W.; Feru, F.; Li, L.; et al. Exploring Targeted Degradation Strategy for Oncogenic KRASG12C. Cell Chem. Biol. 2020, 27, 19–31.e6.
- Bond, M.J.; Chu, L.; Nalawansha, D.A.; Li, K.; Crews, C.M. Targeted Degradation of Oncogenic KRASG12C by VHL-Recruiting PROTACs. ACS Central Sci. 2020, 6, 1367–1375.
- Zhou, C.; Fan, Z.; Zhou, Z.; Li, Y.; Cui, R.; Liu, C.; Zhou, G.; Diao, X.; Jiang, H.; Zheng, M.; et al. Discovery of the First-in-Class Agonist-Based SOS1 PROTACs Effective in Human Cancer Cells Harboring Various KRAS Mutations. J. Med. Chem. 2022, 65, 3923–3942.
- Liu, J.; Xue, L.; Xu, X.; Luo, J.; Zhang, S. FAK-targeting PROTAC demonstrates enhanced antitumor activity against KRAS mutant non-small cell lung cancer. Exp. Cell Res. 2021, 408, 112868.
- Qu, X.; Liu, H.; Song, X.; Sun, N.; Zhong, H.; Qiu, X.; Yang, X.; Jiang, B. Effective degradation of EGFRL858R+T790M mutant proteins by CRBN-based PROTACs through both proteosome and autophagy/lysosome degradation systems. Eur. J. Med. Chem. 2021, 218, 113328.
- Zhao, H.-Y.; Wang, H.-P.; Mao, Y.-Z.; Zhang, H.; Xin, M.; Xi, X.-X.; Lei, H.; Mao, S.; Li, D.-H.; Zhang, S.-Q. Discovery of Potent PROTACs Targeting EGFR Mutants through the Optimization of Covalent EGFR Ligands. J. Med. Chem. 2022, 65, 4709–4726.
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Áñez, G.; Adelglass, J.M.; Hernández, A.Q.B.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F.; et al. Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543.
- Loquai, C.; Hassel, J.C.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Gold, M.; Schwarck-Kokarakis, D.; Attig, S.; Cuk, K.; Vogler, I.; et al. A shared tumor-antigen RNA-lipoplex vaccine with/without anti-PD1 in patients with checkpoint-inhibition experienced melanoma. J. Clin. Oncol. 2020, 38, 3136.
- Xie, Y.J.; Dougan, M.; Jailkhani, N.; Ingram, J.; Fang, T.; Kummer, L.; Momin, N.; Pishesha, N.; Rickelt, S.; Hynes, R.O.; et al. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proc. Natl. Acad. Sci. USA 2019, 116, 7624–7631.
- Ribeiro, R.S.G.; Belderbos, S.; Danhier, P.; Gallo, J.; Manshian, B.B.; Gallez, B.; Bañobre, M.; de Cuyper, M.; Soenen, S.J.; Gsell, W.; et al. Targeting tumor cells and neovascularization using RGD-functionalized magnetoliposomes. Int. J. Nanomed. 2019, 14, 5911–5924.
- Available online: https://clinicaltrials.gov/ct2/show/NCT02996214 (accessed on 7 December 2022).
- Goldinger, S.M.; Dummer, R.; Baumgaertner, P.; Mihic-Probst, D.; Schwarz, K.; Hammann-Haenni, A.; Willers, J.; Geldhof, C.; Prior, J.O.; Kündig, T.M.; et al. Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8+ T-cell responses in melanoma patients. Eur. J. Immunol. 2012, 42, 3049–3061.
- Available online: https://clinicaltrials.gov/ct2/show/NCT04033354 (accessed on 7 December 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT01792479 (accessed on 7 December 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT02283320 (accessed on 7 December 2022).
- Graziani, S.R.; Vital, C.G.; Morikawa, A.T.; Van Eyll, B.M.; Junior, H.J.F.; Filho, R.K.; Maranhão, R.C. Phase II study of paclitaxel associated with lipid core nanoparticles (LDE) as third-line treatment of patients with epithelial ovarian carcinoma. Med Oncol. 2017, 34, 151.
- Available online: https://clinicaltrials.gov/ct2/show/NCT00729612 (accessed on 7 December 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT00077246 (accessed on 7 December 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT01380769 (accessed on 7 December 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT02667743 (accessed on 7 December 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT04486833 (accessed on 7 December 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT04381910 (accessed on 7 December 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT03088813 (accessed on 7 December 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT02769962 (accessed on 7 December 2022).
- Available online: https://clinicaltrials.gov/ct2/show/NCT03670030 (accessed on 7 December 2022).
- Casaluce, F.; Sgambato, A.; Maione, P.; Ciardiello, F.; Gridelli, C. Emerging mitotic inhibitors for non-small cell carcinoma. Expert Opin. Emerg. Drugs 2013, 18, 97–107.
- Hafezi, S.; Rahmani, M. Targeting BCL-2 in Cancer: Advances, Challenges, and Perspectives. Cancers 2021, 13, 1292.
- Lin, Y.-X.; Wang, Y.; Ding, J.; Jiang, A.; Wang, J.; Yu, M.; Blake, S.; Liu, S.; Bieberich, C.J.; Farokhzad, O.C.; et al. Reactivation of the tumor suppressor PTEN by mRNA nanoparticles enhances antitumor immunity in preclinical models. Sci. Transl. Med. 2021, 13.
- Tabernero, J.; Shapiro, G.I.; Lorusso, P.M.; Cervantes, A.; Schwartz, G.K.; Weiss, G.J.; Paz-Ares, L.; Cho, D.C.; Infante, J.R.; Alsina, M.; et al. First-in-Humans Trial of an RNA Interference Therapeutic Targeting VEGF and KSP in Cancer Patients with Liver Involvement. Cancer Discov. 2013, 3, 406–417.
More
