Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 3 by Conner Chen.
Pyruvic acid has numerous applications in the food, chemical, and pharmaceutical industries. General methods of pyruvic acid production include chemical method and biotechnological methods
- pyruvic acid
- yeast
- E. coli
- biochemical pathways
1. Introduction
Pyruvic acid or Pyruvate (PA) is the final product of the glycolysis pathway and is an important intermediate molecule in protein, fat and carbohydrate metabolism [1]. PA is a three-carbon molecule that functions as a critical step in cell metabolism in aerobic and anaerobic conditions [2]. The two PA molecules produced from glycolysis can be converted into various products, like carbohydrates (through gluconeogenesis), alanine, or ethanol (through fermentation), and fatty acids and energy (through Krebs cycle) [1]. Inside the cells, various metabolic pathways are connected by PA [1]. PA is a transparent, water-miscible liquid having an acetic acid-like aroma under normal conditions. PA and its derivatives are widely employed in the pharmaceutical, cosmetic, food, and other important industries [3][4]. PA is also found in sports nutrition supplements, which help athletes to improve their physical condition and maintain body mass control [5]. PA calcium salts were demonstrated to speed up fatty acid metabolism, while also helping to reduce serum cholesterol levels [6]. PA also possesses antioxidant effects and could be used as a nutraceutical to treat diabetes II [7][8].
At the industrial level, pyruvate is mainly used for the production of L-tyrosine, L-tryptophan, N-acetylneuraminic acid (sialic acid), 3-Dihydroxyphenyl (DOPA), levodopa drug class, etc. [9][10]. Current research shows that the microorganisms that ferment to produce pyruvate are mainly E. coli and yeast. In E. coli, lactate dehydrogenase (ldhA), which is responsible for converting pyruvate into lactic acid, is inactivated, and the highest pyruvate production is 110.0 g/L with a mass yield of 0.87 g/g. In yeast, Pyruvate decarboxylase-negative saccharomyces cerevisiae, through directed evolution, obtained a high-yield pyruvate strain that was C2-independent and glucose-tolerant, with a maximum yield of 135 g/L [11]. The pyruvate/glucose conversion rate was 0.54 g/g. Using 70 g/L NaCl as the selection criterion, the NaCl-resistant mutant Torulopsis glabrataRS23 was screened out through continuous culture with pH control. The yield of pyruvate reached 94.3 g/L, and the mass yield was 0.635 g/g. Most of the above-mentioned strains with high pyruvate production use glucose as a substrate, and achieve high production of pyruvate by reducing the consumption of pyruvate. However, pyruvate production mainly uses a chemical synthesis method, which increases its cost and greatly limits its application. In past decades, different microbial strains were generated to enhance their pyruvate production capability.
As an intermediate in the synthesis of many drugs and pesticides, pyruvic acid has a very broad market prospect. However, there is a big gap in the domestic and foreign markets for pyruvic acid, especially for high-quality pyruvic acid. The traditional tartaric acid synthesis has its natural disadvantages. In recent years, there has been rapid development in the field of bioengineering, especially the emergence of gene editing technology, represented by CRISPR/Cas9, which has given more options to modifying microorganisms at the genetic level. Some yeasts, such as Y. lipolytica, have high homologous recombination efficiency. They are relatively easy to perform gene editing operations on so as to facilitate heterologous expression of related genes. In this way, some microorganisms can better combine the natural metabolic pathway of pyruvate. In becoming a natural strain for pyruvic acid production, this is more conducive to improving the production efficiency of pyruvic acid. In addition, with the cross development of genetic engineering, molecular biology, systems biology and other disciplines, rational research on microbial metabolic pathways and gene expression regulation networks has been promoted. Metabolic engineering is also gradually turning towards the development of dynamic regulation, such as cell self-induced dynamic regulation, through metabolite response elements or quorum sensing elements, or artificial control of physical or chemical signals to stimulate promoters and other elements to regulate the expression of downstream genes, providing more reliable theoretical support and technical means for dynamic regulation of microbial fermentation to produce pyruvate.
2. General Methods of Pyruvic Acid Production
2.1. Chemical Method
At present, the synthesis methods of pyruvic acid are mainly chemical methods, such as the tartaric acid method [3], the lactic acid oxidation method and other methods. Among the methods, the tartaric acid method mixes and heats tartaric acid with potassium pyrosulfate, and, finally, the tartaric acid is dehydrated and decarboxylated to form pyruvic acid. According to the latest market situation in 2022, the price of tartaric acid alone reaches about $3000/t. The most important thing is that this method produces serious environmental pollution. Another method uses lactic acid as a raw material and causes it to react with oxygen under the action of a catalyst to produce pyruvic acid. This is a chemical synthesis method, used in many current research works. As the C–C bond is easy to break, producing acetaldehyde and CO2, during the catalytic oxidation of lactic acid, the research is mainly carried out in the comparison and selection of catalysts. With the Pb/Pd/C system as the catalyst [12], the yield of pyruvic acid is relatively ideal. The conditions required for the reaction are mild, green and environment-friendly, and oxygen can be replaced with air in industrial production, which has been gradually adopted by more and more factories in the world.
2.2. Biotechnological Methods
Compared to the chemical approach for pyruvate production, biotechnological methods, including enzymatic, resting cell and fermentative processes, offer a promising alternative for cost-efficient process development. The basic process is shown in Figure 1.
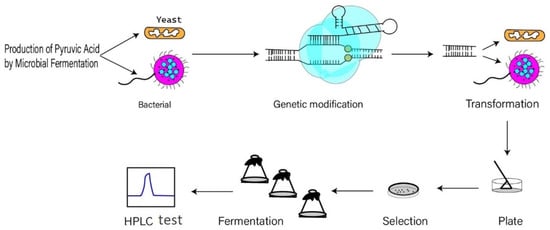
Figure 1. Pyruvic acid production process by microbial fermentation.
2.3. Enzymatic Processes
This process involves a single step carried out by either purified or raw enzymes (or, sometimes, whole cells). Acetobacter sp. has been used to produce about 20 g/L pyruvate by oxidizing D-lactate at a high conversion rate. However, this process is difficult to commercialize, as D-lactate costs more than L-lactate. Another process for pyruvate production involves oxidation of L-lactate to pyruvate by using glycolate oxidase (obtained from Hansenula polymorpha. EC:1.1.3.15) [13].
2.4. Resting Cell Processes
Resting cell processes involve a series of enzymatic steps in non-growing microbial cells provided with a substrate, such as glucose. Acinetobacter sp. and Debaryomyces cerevisiae have been used to produce pyruvate by the resting cell method. This method consumes less time than the fermentation one; however, it involves different steps like cell cultivation, separation from the medium and washing. However, few resting cell methods are performed without separating cells from the medium. By controlling pH or nitrogen source, cell growth can be inhibited. The major issues associated with the extended resting cell processes, includes strain stability and contamination.
2.5. Fermentation Processes
Direct fermentation processes have also been used to produce pyruvate. Many recombinant microorganisms, including yeast such as Y. lipolytica and E. coli, have been developed to accumulate pyruvate from different carbon sources. For example, as an oil producing yeast, Y. lipolytica has a relatively complete metabolic network in its body, so it can use gene editing technology to flow more metabolic flux into the production of pyruvate.
References
- McCommis, K.S.; Finck, B.N. Mitochondrial pyruvate transport: A historical perspective and future research directions. Biochem. J. 2015, 466, 443–454.
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033.
- Li, Y.; Chen, J.; Lun, S.Y. Biotechnological production of pyruvic acid. Appl. Microbiol. Biotechnol. 2001, 57, 451–459.
- Soma, Y.; Tsuruno, K.; Wada, M.; Yokota, A.; Hanai, T. Metabolic flux redirection from a central metabolic pathway toward a synthetic pathway using a metabolic toggle switch. Metab. Eng. 2014, 23, 175–184.
- Jager, R.; Metzger, J.; Lautmann, K.; Shushakov, V.; Purpura, M.; Geiss, K.R.; Maassen, N. The effects of creatine pyruvate and creatine citrate on performance during high intensity exercise. J. Int. Soc. Sport. Nutr. 2008, 5, 4.
- Han, H.S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218.
- Ju, K.D.; Shin, E.K.; Cho, E.J.; Yoon, H.B.; Kim, H.S.; Kim, H.; Yang, J.; Hwang, Y.-H.; Ahn, C.; Oh, K.-H. Ethyl pyruvate ameliorates albuminuria and glomerular injury in the animal model of diabetic nephropathy. Am. J. Physiol.-Ren. Physiol. 2012, 302, F606–F613.
- Plotnikov, E.; Losenkov, I.; Epimakhova, E.; Bohan, N. Protective effects of pyruvic acid salt against lithium toxicity and oxidative damage in human blood mononuclear cells. Adv. Pharm. Bull. 2019, 9, 302–306.
- Cybulski, K.; Tomaszewska-Hetman, L.; Rakicka, M.; Juszczyk, P.; Rywińska, A. Production of pyruvic acid from glycerol by Yarrowia lipolytica. Folia Microbiol. 2019, 64, 809–820.
- Maleki, N.; Eiteman, M.A. Recent progress in the microbial production of pyruvic acid. Fermentation 2017, 3, 8.
- Descotes, J. The popliteal lymph node assay: A tool for studying the mechanisms of drug-induced autoimmune disorders. Toxicol. Lett. 1992, 64–65, 101–107.
- Yang, q.; Yi, X.; Guo, l. Synthesis of Pyruvic Acid from Lactic Acid by Oxidation with Oxygen. Dep. Chem. 2002, 43, 307–309.
- Gao, C.; Ma, C.; Xu, P. Progress in biotransformation of bio-based lactic acid. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2013, 29, 1411–1420.
More
