The genus Lycium belongs to the Solanaceae family and comprises more than 90 species distributed on diverse continents. Lycium barbarum is by far the most studied and considered to possess healthy properties. In contrast, L. europaeum, L. intricatum, and L. schweinfurthii found particularly in the Mediterranean region, are poorly studied, although used by native populations. The biological properties of L. barbarum fruits are mainly attributed to polysaccharides, particularly complex glycoproteins with different compositions. Studies In contrast,regarding these metabolites are practically absent in L. europaeum, L. intricatum, and L. schweinfurthii. foundIn particularlyL. europaeum, in the Mediterranean region, are poorly studied, although used by native populations. The evaluation of the chemical composition and biological, nutritional, or pharmacological properties ofmetabolites isolated and identified belong mainly to polyphenols, fatty acids, carotenoids, sterols, terpenoids, tocopherols, and alkaloids, whereas in L. schweinfurthii, these species must be unveiled. Such studies will not only enrich knowledge but may also lead to the use of some of these species in food to replacemetabolites isolated belong to the phenolic acids, lignans, flavonoids, polyketides, glycosides, terpenoids, tyramine derivatives L. barbarum amorng other plant species. Sincefew compounds; and L. europaeum,for L. intricatum, L. infaustumthe metandbolites L. schweinfurthiicomprise generally occur in impoverished areas, the culture and transformation of these ssters of phenolic acids, glycosides, fatty acids, terpenoids/phytosterols, among other few compounds. Some biological propecrties prattributed to these species include antioxiducts could contribute to the sustained enrichment of the populations living in those zoant, anti-inflammatory and cytotoxic against some cancer cell lines.
- biological properties of Lycium genus
- chemical properties of Lycium genus
- Lycium barbarum
1. Lycium barbarum L.Introduction
Berries are generally known as small edible fruits brightly colored in shades of red, blue, and purple due to the presence of anthocyanins. However, not all berries contain anthocyanins, such as wolfberries or goji (Lycium barbarum L.) [1].
Lycium species are shrubs or small trees, often showing thorns on the stem and leaves, which can be found in the arid and semi-arid regions of North and South America, Africa, and Eurasia [2]. In China, there are seven species and three taxonomic varieties, distributed in Gansu, Qinghai Provinces, Xinjiang, and Ningxia Autonomous regions [3]. It has been hypothesized that the genus Lycium originated in South or North America, and then dispersed to southern Africa, and the Eurasian and Australian species of Lycium are from southern Africa [4].
Lycium barbarum L. is a perennial deciduous shrub that can be found in arid and semi-arid regions of northwestern China, and in southeastern Europe and Mediterranean areas [5,6]. The fruit of this species is known as wolfberry or goji berry, which is orange-red in color, ellipsoid, approximately 2 cm deep, and with a sweet-and-tangy flavor [7]. It is listed in the Traditional Chinese Pharmacopeia [6]. It is also traditionally used in Korean, Japanese, and Vietnamese medicine [8]. The Ningxia Hui autonomous region of China is considered the global birthplace of the wolfberry. However, the cultivation of the species has grown a lot and the great concern of the producers is to guarantee the quality of fruits according to their distinct geographical origin. Therefore several physicochemical methods have been developed generally combined with statistical analysis to discriminate the wolfberries beyond color, shape, odor, taste, and other qualities highly dependent on personal sensorial impressions [10-17].
The importance of this culture has led to the development of mechanized harvesting technologies that accelerate the harvesting process without damaging the fruits, and robots able to replace manual labor [18–20]. In addition, fresh wolfberries are perishable with high water content deteriorating quickly after harvesting, by the microbial attack and mechanical damage [21]. In this context, several strategies have been developed to increase the shelf life of fresh fruits without losing quality [8, 21-23]. However, the dried berries are more popular than the fresh ones, because they can be preserved for longer periods with minimal chemical deterioration and microbial spoilage. Therefore, several drying technologies have been checked beyond the solar drying or hot air [24-34]
Polysaccharides are the most significant metabolites in wolfberries with several biological properties (antioxidant, hepatoprotective, anti-inflammatory, cardioprotective effect, hypoglycemic and immune activities, among other biological attributes) [35–37]. They are generally complex glycoproteins with different composition, although the monosaccharides are generally the same (rhamnose, arabinose, mannose, xylose, galacturonic, glucose, and galacturonic acid) [38]. The polysaccharides are complex, with an approximate molecular weight (MW) of 10–2300 kDa, is mostly composed of (1→3)-β-D-galactopyranosyl, (1→6)-β-D-galactopyranosyl, and (1→4)-α-D-galactopyranosyluronic acid residues. A glycan-O-Ser glycopeptide structure has been mostly considered for the efficacy of L. barbarum as well as 2-O-(β-D-glucopyranosyl) ascorbic acid (could serve as a stable vitamin C substitute) [36,39,40]. Other organic and inorganic components have been detected in wolfberries: carotenoids [40], polyphenols and their derivatives, monoterpenes, alkaloids and spermidine alkaloids [38,41,42], vitamins, dietary fibers, diverse minerals, fatty acids, amino acids and non-protein amino acids [43]. The chemical profile of these berries is dependent on environmental conditions, pre- and post-harvest factors, rhizosphere bacterial community structure and genetic heritage [43,44].
The biological properties of wolfberries have been largely reported and reviewed (antioxidant, anti-inflammatory, anti-aging, hypoglycemic and hypolipidemic activities, modulation of gut microbiota, neuroprotective, neuroprotective effects on retinal ganglion cells, immunomodulatory, positive effects on cognitive impairment, anti-fatigue effect, hepatoprotective, wound healing, and anti-tumor effect [45–63]. There are many scientific studies involving the chemical composition, biological properties, conservation, and drying methods of theame cannot L. barbarum fruit, resulting in more than 20 review articles in indexed scientific journals, in 2022. The same cannot be observed for other species, such as L. europaeum, L. intricatum and L. schweinfurthii. TFor the chemical profile and biological properties of tis reason, this review intends to find and compile information about these species with the aim of awakening interest in these species predominantly found that grow naturally in several Mediterranean countries and Portugal, and that have been scarcely studied.
2. Other Lycium Snot been properly expecloies
Accorteding to the review made by Yao et al. [2], therecommercially as with are four species of Lyci. barbarum.
2.1. that Lycan be found in several places of Europa, Asia, America and Africaium europaeum L. One example is
L.ycium europaeum L.is that can be found in Portugal, Spain, France, Israel, Palestinian Territory, India, Algeria, Tunisia, and Egypt. Lycium schweinfurthii Damma spiny shrub, 1–4 m tall, with red fruits (Figure 1), oblancer is olanother species reported by Yao et al. [2] as being detected in Portugal but also in Spain, Israel, Morocco, Greece, Algeria, Egypt, Tunisia, Mauritania, and Cyprus; and L. intricatum Bote leaves (20–50 × 3–10 mm), calyx (2–3 mm) 5-dentate or 2-lipped, corol1a narrowly infundiss has been was registered in Portugal, Spain, Italy, Morocco, Algeria, Tunisia, Egypt, Mauritania, Saudi Arabia, and Mexico [2]. Despite this relatively wide distribution across several countries and continents, there is very little research on the chemical composition of the various organs of these species as well as their biological or pharmacological propertiesuliform (11–13 mm), pink or white, with stamens usually exserted. Flowers can be solitary or in clusters of two [2].

Figure 1. Lycium infeuropaustueum MiersL. grows in several countries of South America (Argentina, Colombia, Bolivia, Ecuador, Peru, Paraguay), Central America (Dominican, Turks and(Source: https://www.plantarium.ru/lang/en/page/image/id/560323.html) (accessed Caicos Islands, and Jamaica), and Portugal [n 24 September 2022).
2].1.1. The application of L. infaustum aChemical Compos food or medicine is unknowtion and no record could be found, thereby making any type of revision unfeasible in the present review.Biological Properties
2.1. Lycium europaeum L.
So far, only very few dozen of constituents were identified in L. europaeum, which are distributed by the severafollowing groups: polyphenols, fatty acids, polysaccharides, carotenoids, sterols, terpenoids, tocopherols, and alkaloids [64, 65] (Figure 2).
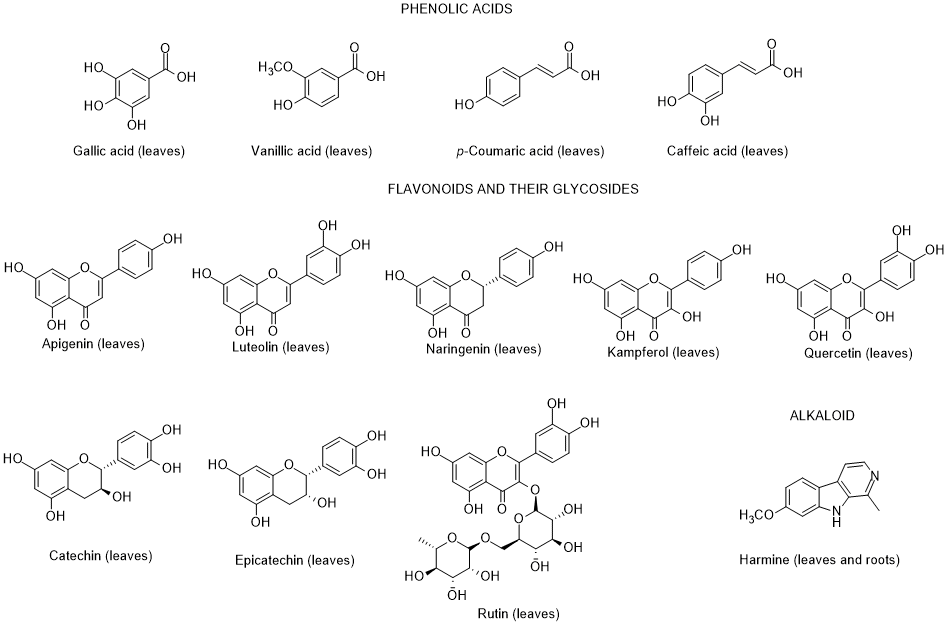
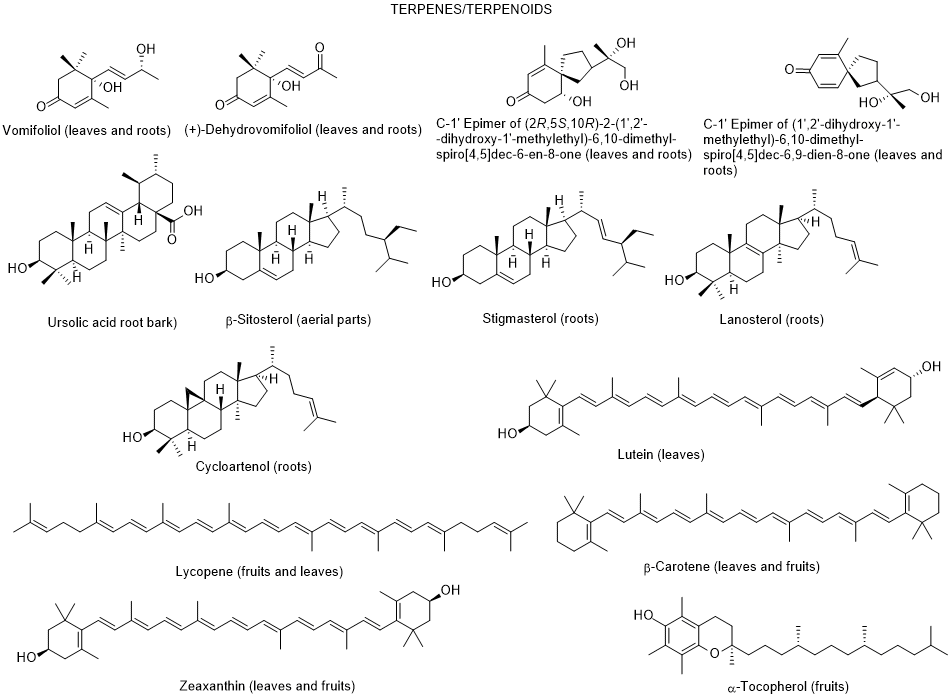
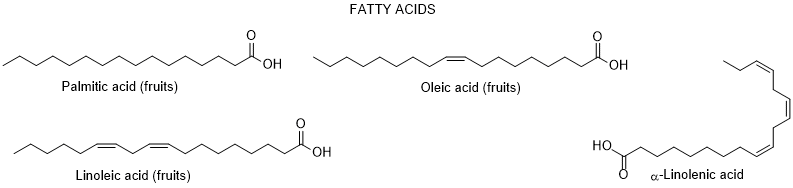
Figure 2. Metabolites found in different parts of Lycium europaeum.
There are diverse biological properties attributed to this species such ((as antioxidant, antinociceptive, hepatoprotective, nephroprotective, hypolipidemic, and cytotoxicity activities), nevertheless without a correspondence between the constituents identified and the biological properties was not established [66]. During 2022, there were no appreciable advances in chemical and biological activities of L. europaeum, only some records focusing: a) ethnobotanical studies; (b) detection for the first time of an infection by a specific fungus of Lycium species in Iran; (c) in vitro antioxidant, anti-acetylcholinesterase, anti-butyrylcholinesterase and anti-urease activities of crude extracts and their fractions; and d) antioxidant and anti-inflammatory activities of fruit methanolic extracts, and e) chemical and digestibility qualities of preferred forage species by lactating Somali camels in Kenya [67-73]. In addition, it was also found a study reporting an infection of L. europaeum by the fungus Arthrocladiella mougeotii (Ascomycota, Helotiales) in Iran, which infects various species of Lycium worldwide [74].
2.2. Lycium schweinfurthii Dammer
Yao et al. [2] reported that leaves and fruits of L. schweinfurthii are used for stomach ulcer. More recently, Ajjoun et al. [75] reported that in Morocco, the decoction of the whole plant is used for hair care; nevertheless, after consulting the original source of information [76], that application is attributed to L. intricatum Boiss. Ajjoun et al. [75] considered L. intricatum as synonymous with L. schweinfurthii. This fact may le chad to the consideration that L. intricatum is the species used as hair care and not L. schweinfurthii. In fact, morphologically, L. schweinfurthii (Figure 3) and L. intricatum (Figure 4) are quite similar with the exception of color of the ripe fruits of L. schweinfurthii which are black, whereas in L. intricatum, the color of ripe fruits are red [77].
|
|
|
|
(a) |
(b) |
Figure 3. Lycium schweinfurthii Dammer: (a) general aspects osf the aerial parts (source: https://www.plantarium.ru/lang/en/page/image/id/652684.html) (accessed on 24 September 2022); and (b) general aspects of fruits (source: https://www.plantarium.ru/lang/en/page/image/id/627979.html).
|
|
|
|
(a) |
(b) |
Figure 4. Lycium intricatum Boiss: (a) general aspects of the aerial parts (source: https://jb.utad.pt/multimedia/10921 (accessed on 24 September 2022); Imagem da espécie Lycium intricatum do Botânico UTAD, Flora Digital de Portugal.); and (b) general aspects of fruits and leaves (https://jb.utad.pt/multimedia/15805); Imagem da espécie Lycium intricatum por Xaverier Béjar do Jardim Botânico UTAD, Flora Digital de Portugal.) https://www.plantarium.ru/lang/en/page/image/id/627979.html).
L. schweinfurthii can also be part found in several places in Palestine, being used for stomach ulcer treatment [78-80].
2.2.1. Chemical Composition oand Biological Properties
The chemical composition of diverse parts of L. schweinfurthii is scarce, nevertheless it has been identified flavonoids and their glycosides, phenolic acids, terpenes including phytosterols and their glycosides, and tyramine derivatives in different parts (roots, stems, leaves, and flowers) of L. schweinfurthii (Figure 5) [74-7781-84]. Regarding the biological properties, soSome compounds (diosmetin, kaempferol, gallic acid and vaginatin) were potent cytotoxic on a skin cancer (G-361) cell line, whereas for colon cancer HCT-116 cells, apigenin was the most effective. Apigenin and diosmetin were the most active against the colon cancer CaCo-2-cells [7784]. Nevertheless, the authors also found that the dichloromethane extract was much more toxic towards G-361 cell line than the isolated compounds, indicating a possible synergism effect among the constituents present in the extract [7784]. Diosmetin, luteolin, quercetin and 3-methoxy-4-O-β-D-glucopyranosyl-methylbenzoate had a potent α-glucosidase inhibitory activity [7784]. According to these results, it can be suggested that this species can be applied as a supplement or even as a medicine, after adequate studies, and not only for use as fuel after cutting as it is currently used, at least in Egypt [85].
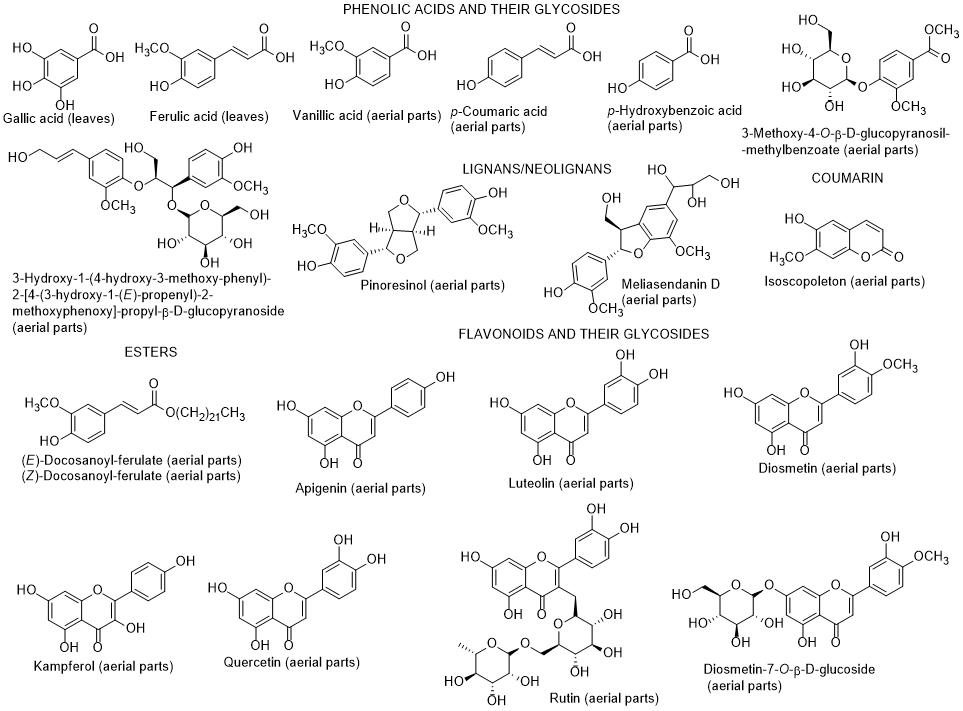
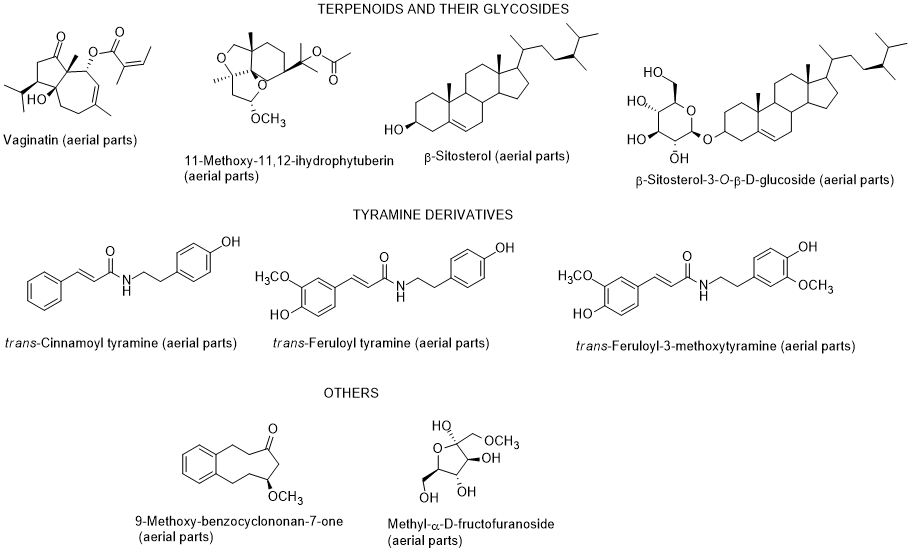
Figure 5. Metabolites found in different parts of Lycium schweinfurthii.
2.2.2. Biotechnological Production of Secondary Metabolites
Taking into account the secondary metabolites previously found by diverse authors for the Egyptian L. schweinfurthii [76-7983–86], Mamdouh and Smetanska [7986] aimed to obtain callus and cell suspension cultures of this species for use in bio-factories for secondary metabolites producing secondary metabolites with biologition, because it could provide a cost-effective alternative to traditional cultivation [87]. For the optimization, diverse factors were evaluated such as plant growth regulators and their combinations in the Murashige and Skoog medium (MS) for calli and N2 for caell propertiessuspension culture. In the suspensions, the authors studied the effect of diverse concentrations of sucrose on the growth and secondary metabolites production [86]. In vitro production of micropropagated plants was another biotechnological approach to produce secondary metabolites (phenols such as ferulic acid) with antioxidant activity [888].
2.2.3. Secondary Metabolites from Endophytic Fungus
More recently, Elbermawi et al. [89] isolated from the fresh leaves of L. schweinfurthii in Egypt, an endophytic fungus identified as Alternaria sp., which after growing on solid rice culture media were able to produce phenolic compounds (talaroflavone, alternarienoic acid, altenuene, altenusin, alternariol and alternariol-5-O-methyl ether) (Figure 6). Alternarienoic acid, altenuene and altenusin had potent in vitro α-glucosidase and pancreatic lipase inhibitory activities. The molecular docking study was done to predict the preferred fitting between two of the interacting chemical moieties of the phenolic compound and protein, using a computational simulation. The authors concluded that alternarienoic acid, altenuene and altenusin showed a maximum number of interactions with amino acids residues in the active site [89]. According to the authors, these inhibitory activities can be promising naturally occurring anti-diabetic candidates, nevertheless some of these phenolic polyketides are mycotoxins and they present genetic, reproductive, and developmental toxicity [90]. As such, those type of conclusions should be considered with care.
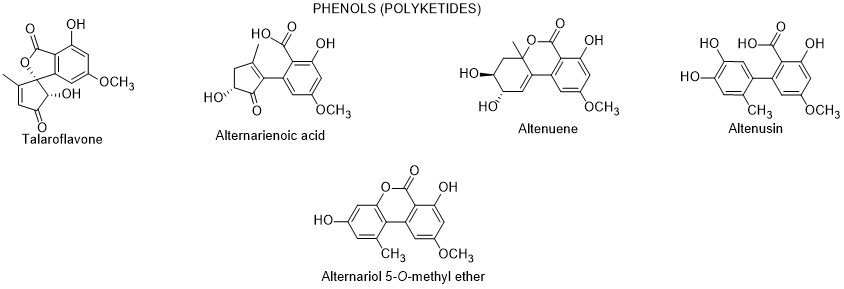
Figure 6. Metabolites isolated from the endophytic Alternaria sp. isolated from the leaves of L. schweinfurthii.
2.3. Lycium intricatum Boiss.
Lycium intricatum can occur in open termophilic halo-nitrophilous matorrals, next to the coastal dunes, in calcareous and saline soils [91], making it a salt-tolerant plant [92]. According to these authors, this species has been considered important for the success of restoration programs in dry environments and also for hedge and wind break plant purposes. However, L. intricatum was heavily depleted because it was systematically used for firing the kilns in a specific zone of the arid island Fuerteventura (Spain) [93]. In addition, it was observed that this species was able to accumulate heavy metals (As, Cd, Cu, Fe, Pb, and Zn) in their shoots and roots without being affected by excessive metal contents. As so, the authors [94] suggested that L. intricatum could be used to reduce metals dissemination by erosion or leaching, with the advantage of being well accepted by populations.
2.3.1. Chemical Composition
Seeds of L. intricatum have been used in helminthiasis, as a digestive, whereas the fruits have been used in eye diseases [2]. A decoction made with leaves have been used in stomach pain and intestinal diseases, with a relative strong Fidelity Level (72%) [95] One citation was reported by the ethnobotanical study made by Fatiha et al. [96] for the utilization of L. intricatum in some genitourinary ailments in the Middle Oum Rbia (Morocco).
In 2015, and for the first time, Abdennacer et al. [8197] reported that polyphenols, including flavonoids, predominate in L. intricatum leaves collected in Tunisia. Nineteen phenolic compounds were isolated and fifteen were identified (dicaffeoylquinic acid isomers, chlorognic acid, dicaffeoylputrescine, caffeoylputrescine, mono-caffeoylquinic acid, p-coumaroylquinic acid, feruloylquinic acid, rutin, isoquercitrin, quercetin dirhamnoside, quercitrin, kampferol rutinoside, isorhamnetin glucoside) (Figure 7). Only chlorogenic acid, caffeoylputrescine, p-coumaroylquinic acid, feruloylquinic acid and rutin could be detected in both leaves and fruits. Although the authors reported that anthocyanins predominate in fruits, they did not present the identification of any anthocyanin. The absence of structure elucidation of the anthocyanins quantified by Abdennacer et al. [8197] must be the object of further studies, since so far anthocyanin identification in fruits of Lycium species were only reported in L. ruthenicum [982].
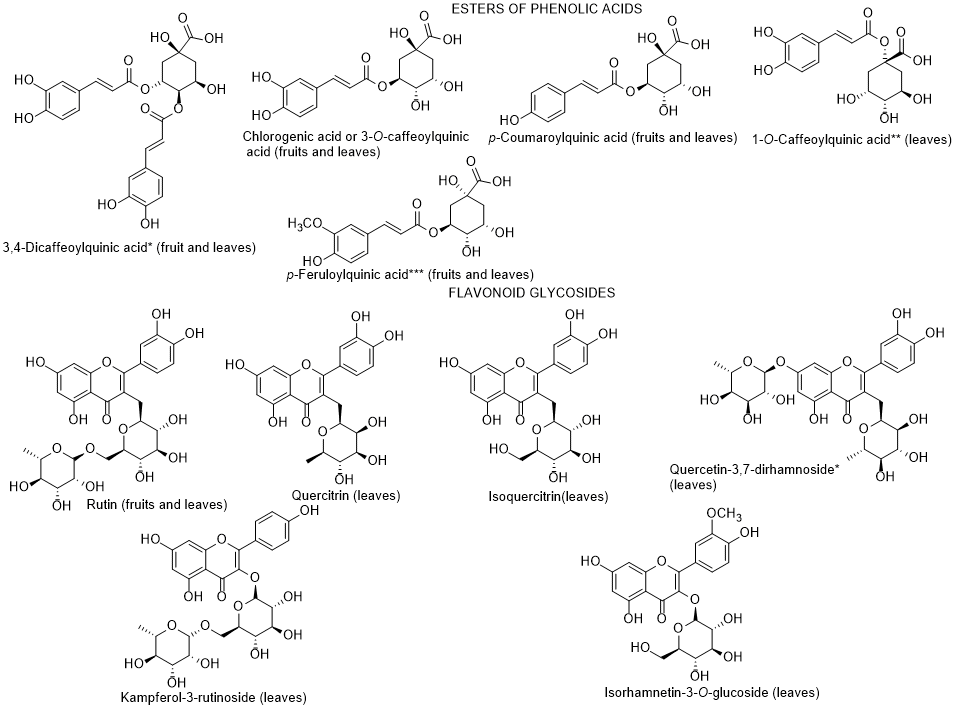
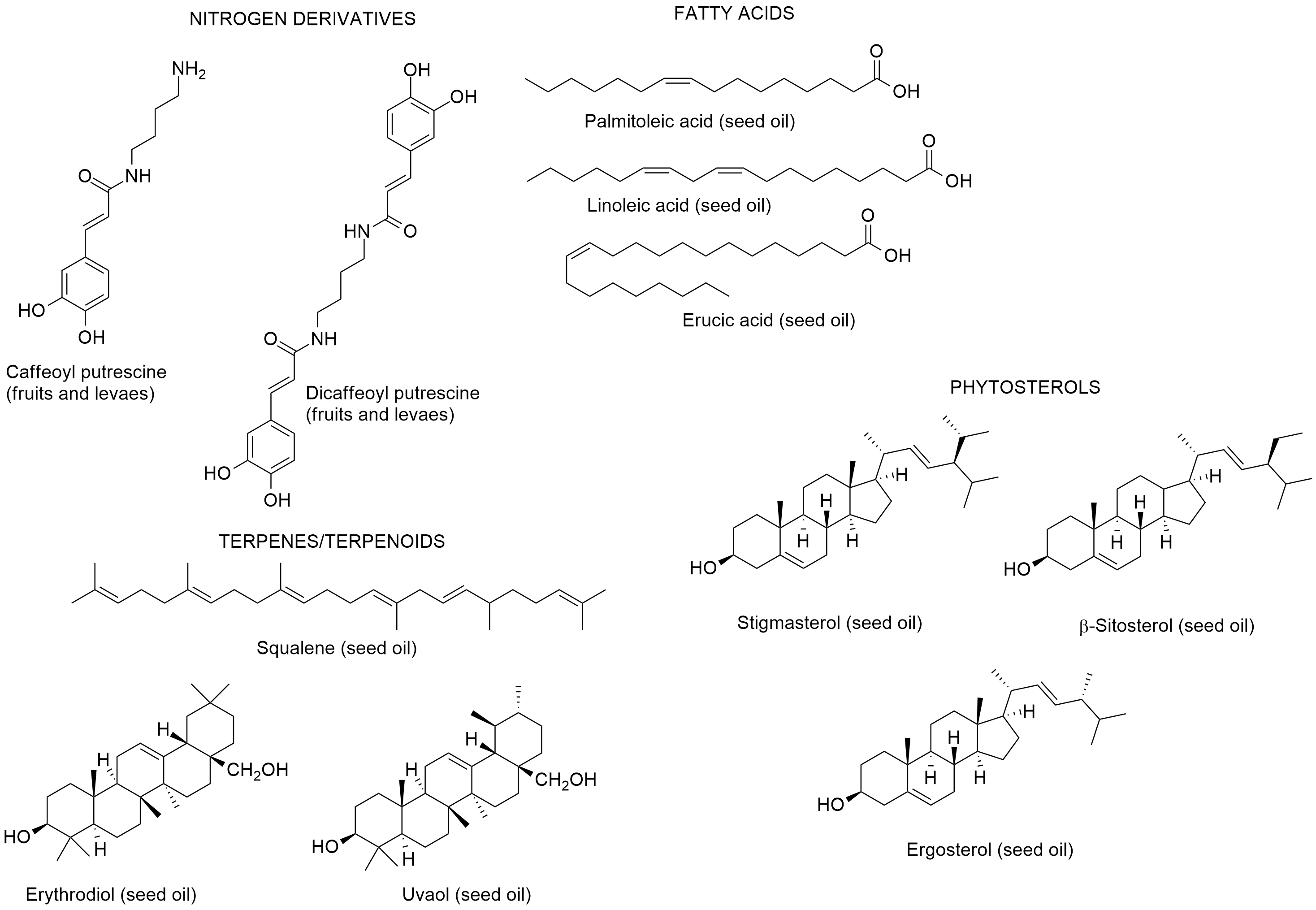
Figure 7. Metabolites found in different parts of Lycium intricatum. *, **, *** The isomers were not reported by the authors [96], therefore, the compounds represented are examples and not the isomers identified.
Boulila and Bejaoui [99], reported the chemical composition of the seed oil of L. intricatum from Northern Tunisia. Linoleic, pater on, lmitoleic and erucic acids were the main fatty acids; and the hydrocarbon squalene, and the triterpenic alcohols erythrodiol and uvaol were also found in the seed oil. The sterolic fraction had stigmasterol, β-sitosterol and ergosterol. Later on, Bendjedou et al. [83100] reported new compounds in leaf extracts of L. intricatum from Algeria: (1R,3aR,7aS)-3a,7-dimethyl-1-(E)-prop-1-en-1-yl-1,3a,4,7a-tetrahydroisobenzofuran-5(3H)-one; isoscopoletin; 3,4,5-trimethoxybenzyl alcohol; and (+)-isolariciresinol (Figure 8).
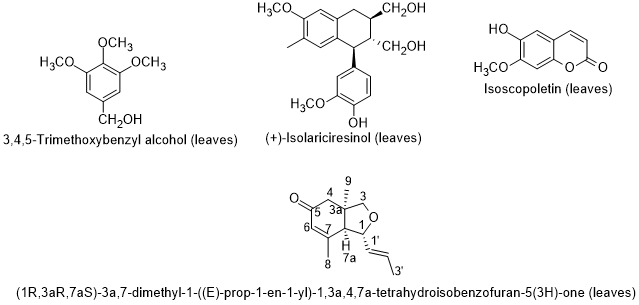
Figure 8. Metabolites Bfoulila andnd in leaf extracts of Lycium intricatum from BAlgejaoui [84],ria [100].
As can rbeported observed, the chemical composition of the seed oilelucidation of diverse parts of L. intricatum is scarce and from Northern Tunisia. Linoleic, palmitonly from Tunisia and Algeria. This means that more studies on the chemical composition of this species spread in the Mediterranean basin are needed.
2.3.2. Secondary Metaboleic ites from Endophytic Fungus
There are compound erucic acids were the main fs produced by endophytic microorganisms that have biological attributes. In review works, the authors [101, 102] reported diverse compounds isolated from endophytic fungus isolated from L. intricatum. Pyrenophorol, dihydropyrenophorin, 4-acetty acids;ylpyrenophorol, 4-acetyldihydropyrenophorin, acis-dihydro-pyrenophorind, theetrahydropyrenophorin, seco-dihydrocapyrbon squalenophorin, 7-acetyl seco-dihydropyreneophorin, and seco-dihydropyrenophorin-1,4-lacthone (Figure triter9) are examples of compounds isolated from Phoma sp., an enic adophytic fungus isolated from L. intricatum [103]. All these coholsmpounds presented antifungal activity against Microbotryum violaceum [104]. Another rythrodeview article refers that the xanthones microsphaeropsones A-C, (Figure 9) isolated from Microsphaeropsis sp., a microorganism associated with L. intricatum [102], had antibacterial and algicidal uvaol were also found in activities [104,102]. However, and previously, other new components were already isolated from endophytic microorganisms in L. intricatum [105] and compiled in the sreceed oil. The sterolic fractionnt review article [106] (Figure 10). Such compounds were isolated from Microdiplodia sp. had stigmastnd possessed activity against Legionella pneumophila.
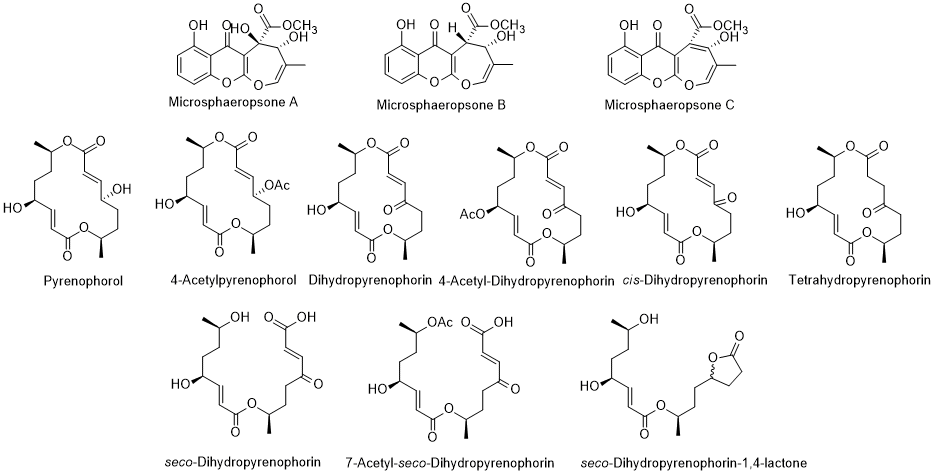
Figure 9. Mertabol, β-sitoites isolated from endophytic Microsphaeropsis sp. (therol first three compounds) and Phoma sp. isolated frgom L. intricatum.
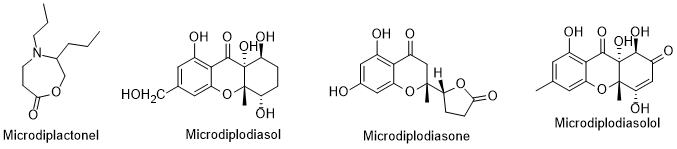
Figure 10. Metabolitesterol.
S isolated from eendophytic Microdiplodia sp. ofisolated from L. intricatum.
3. Conclusions
Among the Lycium species, L. barbarum has been extensive been used in helminthiasis, as a digestive, whereas the fruitly studied, particularly the berries The compounds isolated are distributed into diverse compound classes (polysaccharides, glycerogalactolipids, phenylpropanoids, coumarins, lignans, flavonoids, amides, alkaloids, anthraquinones, organic acids, terpenoids, sterols, steroids and their derivatives, and peptides), [36,39,107]. In contrast, significantly fewer studies have been used focused on other Lycium species such as L. europaeum, L. intricatum, L. schweinfurthii and L. infaustum.
Theyre diseases [2]. A decoction made wiis little research on chemical composition and biological properties of L. infaustum (ath leaves hast published in scientific articles). Concerning L. intricatum, very been used in stomach few compounds (nineteen polyphenols, fatty acids, terpenes, and phytosterols) were identified. Nevertheless, recently, three new compounds, 3,4,5-trimethoxybenzyl alcohol, (+)-isolariciresinol and [(1R,3aR,7aS)-3a,7-dimethyl-1-(E)-prop-1-en-1-yl-1,3ain a,4,7a–tetrahydroisobenzofuran-5(3H)-one] were identified in leaves of L. intricatum from Algeria, not yet d inteescribed in other Lycium species.
Very few works about the cheminal cal composition and biological activities of L. schweinfurthii can be found. Diseases, with a relatverse classes of compounds have been identified, which are within those previously summarized for Lycium species [107]. Howeve strong Fider, in samples of Egyptian origin, a new compound of a natural source was identified, 3-methoxy-4-O-β-D-gliucopyranosyl-mety Level (72%) [85] One hylbenzoate, and compounds not yet found in Lycium specites [vaginatin, (E)-docosan waoyl ferulate and (Z)-docosanoyl reported by the ethnobotanical studyferulate, methyl-α-D-fructofuranoside and meliasendanin D]. The above-mentioned new compound was shown to be a potent inhibitor of α-glucosidase activity, along with diosmetin, luteolin and quercetin.
Through a review made by FWatiha et al. [86] for the utilizationnes and Tounsi [64], in 2021, it was possible to determine that only 30 constituents could be identified in of L. inteuricatopaeum, in some genitoudistributed by the same classes already reported for Lycium barbarum [107]. Diverse binary ailmentsological attributes were also reported in the Middle Oum Rbia (Morocco).
Tat compilation, including: antioxidant, anti-inflammatory, antinociceptive, hypoglycemic, hypolipide present smic, hepatoprotective, nephroprotective and cytotoxic activities.
4. Future Trendy
This review can be the trigger for the beginning of more in-depth studies on these species with the aim of knowing if they can have the same uses of L. barbarum or even new applications. Since L. europaeum, L. intricatum, L. infaustum and L. schweinfurthii generally occur in impoverished areas, the culture and transformation of these species products could contribute to the sustained enrichment of the populations living in those zones.
Funding: This research was funded by FCT (Fundação para a Ciência e a Tecnologia, Portugal) under the project UIDB/05183/2020.
Conflicts of Interest: The author declares no conflict of interest.
References
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Berries: Anti-inflammatory effects in humans. J. Agric. Food Chem. 2014, 62, 3886–3903.
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66.
- Zhang, D.; Xia, T.; Dang, S.; Fan, G.; Wang, Z. Investigation of Chinese wolberry (Lycium spp.) germplasm by restriction site- associated DNA sequencing (RAD-seq). Biochem. Genet. 2018, 56, 575–585.
- Fukuda, T.; Yokoyama, J.; Ohashi, H. Phylogeny and biogeography of the genus Lycium (Solanaceae): Inferences from chloroplast DNA sequences. Mol. Phylogenet. Evol. 2001, 19, 246–258.
- Zhou, Z.; Xiao, J.; Fan, H.; Yu, Y.; He, R.; Feng, X.; Gao, H. Polyphenols from wolfberry and their bioactivities. Food Chem. 2017, 214, 644–654.
- Xing, L.; Wang, Y.; Luo, R.; Li, X.; Zou, L. Determination of 31 pesticide residues in wolfberry by LC-MS/MS and dietary risk assessment of wolfberry consumption. Food Sci. Tecnol. 2022, 42, e61921. https://doi.org/10.1590/fst.61921.
- Kulczyński, B.; Gramza-Michałowska, A. Gojy berry (Lycium barbarum): Composition and health effects—A review. Pol. J. Food Nutr. Sci. 2016, 66, 67–76.
- Liang, Z.; Luo, Z.; Li, W.; Yang, M.; Wang, L.; Lin, X.; Li, L. Elevated CO2 enhanced the antioxidant activity and downregulated cell wall metabolism of wolfberry (Lycium barbarum L.). Antioxidants 2022, 11, 16. https://doi.org/10.3390/antiox11010016.
- Meng, J.; Liu, Z.; Gou, C.L.; Rogers, K.M.; Yu, W.-J.; Zhang, S.-S.; Yuan, Y.-W.; Zhang, L. Geographical origin of Chinese wolfberry (goji) determined by carbon isotope analysis of specific volatile compounds. J. Chromatogr. B 2019, 1105, 104–112.
- Yin, T.; Guo, T.; Ma, Z.; Wang, Z.; Sun, X.; Li, C. Classification of wolfberry with different geographical origins by using voltametric electronic tongue. IFAC PapersOnLine 2018, 51, 654–659.
- Yang, Z.; Wang, Z.; Yuan, W.; Li, C.; Jing, X.; Han, H. Classification of wolfberry from different geographical origins by using electronic tongue and deep learning algorithm. IFAC Papers OnLine 2019, 52, 397–402.
- Zhang, S.; Wei, Y.; Wei, S.; Liu, H.; Guo, B. Authentication of Zhongning wolfberry with geographical indication by mineral profile. Int. J. Food Sci. Technol. 2017, 52, 457–463.
- Dong, F.; Hao, J.; Luo, R.; Zhang, Z.; Wang, S.; Wu, K. Identification of the proximate geographical origin of wolfberries by two-dimensional correlation spectroscopy combined with deep learning. Comput. Electron. Agric. 2022, 198, 107027. https://doi.org/10.1016/j.compag.2022.107027.
- Du, M.; Gong, Y.; Lin, Z.Z.; Shi, X.Y.; Hua, G.D.; Qiao, Y.J. Rapid identification of wolfberry fruit of different geographic regions with sample surface near infrared spectra combined with multi-class SVM. Spectrosc. Spect. Anal. 2013, 33, 1211–1214.
- Zhong, L.; Liu, M.D.; Ji, S.X. The identification of the origin of Chinese wolfberry based on infrared spectral technology and the artificial neural network. Spectrosc. Spect. Anal. 2016, 36, 720–723.
- Gong, H.; Rehman, F.; Li, Z.; Liu, J.; Yang, T.; Liu, J.; Li, H.; Hu, Z.; Ma, Q.; Wu, Z.; et al. Discrimination of geographical origins of wolfberry (Lycium barbarum L.) fruits using stable isotopes, earth elements, free amino acids, and saccharides. J. Agric. Food Chem. 2022, 70, 2984–2997.
- Bondia-Pons, I.; Savolainen, O.; Törrönen, R.; Martinez, J.A.; Poutanen, K.; Hanhineva, K. Metabolic profiling of Gogi berry extracts for discrimination of geographical origin by non-targeted liquid chromatography couples to quadrupole time-of-flight mass spectrometry. Food Res. Int. 2014, 63, 132–138.
- Zhang, X.; Zhao, Z.; Wei, S.; Du, G.; Ji, Q.; Zhang, L.; Wei, H. Study on the design and control system for wolfberry harvesting robot. In Proceedings of the 28th Chinese Control and Decision Conference, Yinchuan, China, 28–30 May 2016; pp. 5984–5985.
- Wang, J.; Mei, S.; Xiao, G.; Zhou, H. Research on mechanized harvesting methods of Lycium barbarum fruit. IFAC papersOnLine 2018, 51, 223–226.
- Chen, Y.; Zhao, J.; Hu, G.; Chen, J. Design and testing of a pneumatic oscillating Chinese wolfberry harvester. Horticulturae 2021, 7, 214. https://doi.org/10.3390/horticulturae7080214.
- Ban, Z.; Wei, W.; Yang, X.; Feng, J.; Guan, J.; Li, L. Combination of heat treatment and chitosan coating to improve postharvest quality of wolfberry (Lycium barbarum). Int. J. Food Sci. Technol. 2015, 50, 1019–1025.
- Xiang, W.; Wang, H.-W.; Tian, Y.; Sun, D.-W. Effects of salicylic acid combined with gas atmospheric control on postharvest quality and storage stability of wolfberries: Quality attributes and interaction evaluation. J. Food Process Eng. 2021, 44, e13764. https://doi.org/10.1111/jfpe.13764.
- .Ling, L.; Zhao, Y.; Tu, Y.; Yang, C.; Ma, W.; Feng, S.; Lu, L.; Zhang, J. The inhibitory effect of volatile organic compounds produced by Bacillus subtilis CL2 on pathogenic fungi of wolfberry. J. Basic Microbiol. 2021, 61, 110–121.
- Yang, M.; Ding, C.; Zhu, J. The drying quality and energy consumption of Chinese wolfberry fruits under electrohydrodynamic system. Int. J. Appl. Electromagn. Mech. 2017, 55, 101–112.
- Yang, M.; Ding, C. Electrohydrodynamic (EHD) drying of the Chinese wolfberry fruits. SpringerPlus 2016, 5, 909. https://doi.org/10.1186/s40064-016-2546-1.
- Ni, J.; Ding, C.; Zhang, Y.; Song, Z.; Hu, X.; Hao, T. Effect of electrohydrodynamic partially combined with oven drying on Chinese wolfberry. Int. J. Appl. Electromagn. Mech. 2020, 63, 465–482.
- Ni, J.; Ding, C.; Zhang, Y.; Song, Z.; Hu, X.; Hao, T. Electrohydrodynamic drying of Chinese wolfberry in a multiple needle-to-plate electrode system. Foods, 2019, 8, 152. https://doi.org/10.3390/foods8050152.
- Xie, L.; Zengh, Z.-A.; Mujumdar, A.S.; Fang, X.-M.; Wang, J.; Zhang, Q.; Ma, Q.; Xiao, H.-W.; Liu, Y.-H.; Gao, Z.-J. Pulsed vacum drying (PVD) of wolfberry: Drying kinetics and quality attributes. Drying Technol. 2018, 36, 1501–1514.
- Yu, F.; Li, Y.; Wu, Z.; Wang, X.; Wan, N.; Yang, M. Dehydration of wolfberry fruit using pulsed vacuum drying combined with carboxymethyl celulose coating pretreatment. LWT–Food Sci. Technol. 2020, 134, 110159. https://doi.org/10.1016/j.lwt.2020.110159.
- Hu, Z.; Zhang, S.; Chu, W.; He, W.; Yu, C.; Yu, H. Numerical analysis and preliminar experiment of a solar assisted heat pump drying system for Chinese wolfberry. Energies 2020, 13, 4306. https://doi.org/10.3390/en13174306.
- Xie, L.; Mujumdar, A.S.; Fang, X.-M.; Wang, J.; Dai, J.-W.; Du, Z.-L.; Xiao, H.-W.; Liu, Y.; Gao, Z.-J. Far-infrared radiation heating assisted pulsed vacuum drying (FIR-PVD) of wolfberry (Lycium barbarum L.): Effects on drying kinetics and quality attributes. Food Bioprod. Process. 2017, 102, 320–331.
- Qi, Y.; Yu, F.; Wang, X.; Wan, N.; Yang, M.; Wu, Z.; Li, Y. Drying of wolfberry fruit juice using low-intensity pulsed ultrasound. LWT–Food Sci. Technol. 2021, 141, 110953. https://doi.org/10.1016/j.lwt.2021.110953.
- Xu, Y.; Zang, Z.; Zhang, Q.; Wang, T.; Shang, J.; Huang, X.; Wan, F. Characteristics and quality analysis of radio frequency-hot air combined segmented drying of wolfberry (Lycium barbarum). Foods 2022, 11, 1645. https://doi.org/10.3390/foods11111645.
- Cui, C.; Zhao, D.; Huang, J.; Hao, J. Progress on research and development of goji berry drying: A review. Int. J. Food Propert. 2022, 25, 435–449.
- Fan, J.; Lin, L.; Zhao, M. Construction of in vitro fermentation model using gut microbiota relating to glucose and lipid metabolism: A supplementary method for initial screening of polysaccharides with hypoglycemic potentials. J. Sci. Food Agric. 2022, 102, 6328–6339. https://doi.org/10.1002/jsfa.11983.
- Ma, R.-H.; Zhang, X.-X.; Ni, Z.-J.; Thakur, K.; Wang, W.; Yan, Y.-M.; Cao, Y.-L.; Zhang, J.-G.; Rengasamy, K.R.R.; Wei, Z.-J. Lycium barbarum (goji) as functional food: A review of its nutrition, phytochemical structure, biological features, and food industry prospects. Crit. Rev. Food Sci. Nutr. 2022. https://doi.org/10.1080/10408398.2022.2078788.
- Wang, Y.; Jin, H.; Dong, X.; Yang, S.; Ma, S.; Ni, J. Quality evaluation of Lycium barbarum (wolfberry) from different regions in China based on polysaccharide structure, yield and bioactivies. Chin. Med. 2019, 14, 49. https://doi.org/10.1186/s13020-019-0273-6.
- Potterat, O. Goji (Lycium barbarum and L. L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7–19.
- Ma, R.-H.; Zhang, X.-X.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. Research progress of Lycium barbarum L. as functional food: Phytochemical composition and health benefits. Curr. Opin. Food Sci. 2022, 47, 100871. https://doi.org/10.1016/j.cofs.2022.100871.
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium barbarum: A traditional Chinese herb and a promising anti-aging agent. Aging Dis. 2017, 8, 778–791.
- Chen, D.; Guo, S.; Zhou, J.; Zhu, Y.; Zhang, F.; Zeng, F.; Duan, R.; Xu, M.; Duan, J.-A. Chemical constituents from Lycium barbarum (Solanaceae) and their chemophenetic significance. Bioch. Syst. Ecol. 2021, 97, 104292. https://doi.org/10.1016/j.bse.2021.104292.
- Liu, J.; Gong, Y.; Yang, J.; Di, D. Advance on alkaloids of Lycium genus. Chin. Sci. Bull. 2022, 67, 332–350.
- Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Dijuriš, J.D.; Ilić, T.D.; Kostić, A.Z.; Pešić, M.B. Health benefits and applications of goji berries in functional food products development: A review. Antioxidants 2022, 11, 248. https://doi.org/10.3390/antiox11020248.
- Liu, S.Y.; Wang, Q.Q.; Lei, Y.H.; Wang, S.S.; Chen, K.L.; Li, Y.; Xiong, J.; Liang, X.J.; Zhou, X.; Li, Y.K. Elucidating the interaction of rhizosphere bacteria and environmental factors in influencing active ingredient content of Lycium barbarum fruit in China. J. Appl. Microbiol. 2022, 132, 3783–3796.
- Sim, R.H.; Sirasanagandla, S.R.; Das, S.; Teoh, S.L. Treatment of glaucoma with natural products and their mechanism of action: An update. Nutrients 2022, 14, 534. https://doi.org/10.3390/nu14030534.
- Rang, Y.; Liu, H.; Liu, C. Potential for non-starch polysaccharides in the prevention and remediation of cognitive impairment: A comprehensive review. Int. J. Biol. Macromol. 2022, 208, 182–195.
- Qi, Y.; Duan, G.; Fan, G.; Peng, N. Effect of Lycium barbarum polysaccharides on cell signal transduction pathways. Biomed. Pharmacother. 2022, 147, 112620. https://doi.org/10.1016/j.biopha.2022.112620.
- Xiao, Z.; Deng, Q.; Zhou, W.; Zhang, Y. Immune activities of polysaccharides isolated from Lycium barbarum L.. What do we know so far? Pharmacol. Therap. 2022, 229, 107921. https://doi.org/10.1016/j.pharmthera.2021.107921.
- Huang, R.; Wu, E.; Deng, X. Potential of Lycium barbarum polysaccharide for the control of glucose and lipid metabolism disroders: A review. Int. J. Food Prop. 2022, 25, 673–680.
- Pinilla, I.; Maneu, V.; Campello, L.; Fernández-Sánchez, L.; Martínez-Gil, N.; Kutsyr, O.; Sánchez-Sáez, X.; Sánchez-Castillo, C.; Lax, P.; Cuenca, N. Inherited retinal dystrophies: Role of oxidative stress and inflammation in their physiopathology and therapeutic implications. Antioxidants 2022, 11, 1086. doi.org/10.3390/antiox11061086.
- Yang, C.; Zhao, Q.; Li, S.; Pu, L.; Yu, L.; Liu, Y.; Lai, X. Effects of Lycium barbarum L: Polysaccharides on vascular retinopathy: An insight review. Molecules 2022, 27, 5628. doi.org/10.3390/molecules27175628.
- Liu, H.; Cui, B.; Zhang, Z. Mechanism of glycometabolism regulation by bioactive compounds from the fruits of Lycium barbarum: A review. Food Res. Int. 2022, 159, 111408. doi.org/10.1016/j.foodres.2022.111408.
- Rohilla, P.; Jain, H.; Chhikara, A.; Singh, L.; Dahiya, P. Anticancer potential of Solanaceae plants: A review. S. Afr. J. Bot. 2022, 149, 269–289.
- Zhang, Z.; Wang, S.; Tan, H.; Yang, P.; Li, Y.; Xu, L.; Duan, B.; Liu, Y. Advances in polysaccharides of natural source of the anti-Alzheimer’s disease effect and mechanism. Carbohydr. Polym. 2022, 296, 119961. doi.org/10.1016/j.carbpol.2022.119961.
- Xu, W.; Jiang, Y.; Wang, N.; Bai, H.; Xu, S. Traditional Chinese medicine as a promising strategy for the treatment od Alzheimer’s disease complicated with osteoporosis. Front. Pharmacol. 2022, 13, 842101. https://doi.org/10.3389/fphar.2022.842101.
- Liu, Y.; Li, C.; Shen, X.; Liu, Y. The use of traditional Chinese medicines in relieving exercise-induced fatigue. Front. Pharmacol. 2022, 13, 969827. https://doi.org/10.3389/fphar.2022.969827.
- Che, Z.; Zhou, Z.; Xiao, J. Mechanisms of wolfberry in the treatment of liver diseases. Chin. Sci. Bull. 2022, 67, 351–363.
- Ren, Y.; Li, S.; Song, Z.; Luo, Q.; Zhang, Y.; Wang, H. The regulatory roles of polysaccharides and ferroptosis-related phytochemicals in liver diseases. Nutrients 2022, 14, 2303. https://doi.org/10.3390/nu14112303.
- Wang, Y.; Chen, R.; Yang, Z.; Wen, Q.; Cao, X.; Zhao, N.; Yan, J. Protective effects of polysaccharides in neurodegenerative diseases. Front. Aging Neurosci. 2022, 14, 917629. https://doi.org/10.3389/fnagi.2022.917629.
- Feng, Y.; Song, Y.; Zhou, J.; Duan, Y.; Kong, T.; Ma, H.; Zhang, H. Recent progress of Lycium barbarum polysaccharides on intestinal microbiota, microbial metabolites and health: A review. Crit. Rev. Food Sci. Nutr. 2022, https://doi.org/10.1080/10408398.2022.2128037.
- Cao, C.; Wang, Z.; Gong, G.; Huang, W.; Huang, L.; Song, S.; Zhu, B. Effects of Lycium barbarum polysaccharides on immunity and metabolic syndrome associated with the modulation of gut microbiota: A review. Foods 2022, 11, 3177. doi.org/10.3390/foods11203177.
- Wang, B.; Han, L.; Liu, J.-M.; Zhang, J.; Wang, W.; Li, B.-G.; Dong, C.-X.; Bai, C.-C. Lycium genus polysaccharides: An overview of its extraction, structures, pharmacological activities and biological applications. Separations 2022, 9, 197. https://doi.org/10.3390/separations9080197.
- Gong, H.; Li, W.; Sun, J.; Guan, Q.; Guo, Y.; Wang, Y. A review on plant polysaccharide based on drug delivery system for construction and application, with emphasis on traditional Chinese medicine polysaccharide. Int. J. Biol. Macromol. 2022, 211, 711–728.
- Wannes, W.A.; Tounsi, M.S. Phytochemical composition and health properties of Lycium europaeum L.: A review. Acta Ecol. Sinica 2021, 41, 390–401.
- Yao, X.; Peng, Y.; Xu, L.-J.; Li, L.; Wu, Q.-L.; Xiao, P.-G. Phytochemical and biological studies of Lycium medicinal plants. Chem. Biodivers. 2011, 8, 976–1010.
- Akter, F.; Rahman, M.S.; Al Amin, G.M.; Miah, M.I.; Koh, Y.-S. Beneficial therapy with natural anti-inflammatory agents and supplements. J. Bacteriol. Virol. 2021, 51, 149–162.
- Ikanya, L.W.; Maina, J.G.; Gachuiri, C.K.; Owino, W.O.; Dubeux, J.C.B., Jr. Chemical composition, and digestibility of preferred forage species by lactating Somali camels in Kenya. Rangel. Ecol. Manag. 2022, 80, 61–67.
- Chaachouay, N.; Azeroual, A.; Bencharki, B.; Douira, A.; Zidane, L. Ethnoveterinary medicinal plants for animal therapy in the Rif, North of Morocco. S. Afr. J. Bot. 2022, 147, 176–191.
- Mollel, N.P.; Otieno, J.N.; Sitoni, D.K. Medicinal plants traded in Arusha city, Tanzania. J. Med. Plants Stud. 2022, 10, 175–182.
- El-Mokasabi, F. Survey of wild trees and shrubs in Eastern region of Libya and their economical value. Alq. J. Med. App. Sci. 2022, 5, 48–55.
- Almushghub, F.; Ahmed, D.; El-Din, A.S.; Shaltout, K. Vegetation analysis of Wadi Kaam at northwest Libya. J. Basic Environm. Sci. 2022, 9, 20–37.
- Bendjedou, H.; Bennaceura, M.; Benamar, H.; Rodrigues, M.J.; Pereira, C.; Bensouicie, C.; Custódio, L. In vitro antioxidant and enzyme inhibitory properties and phenolic contents of crude extracts and fractions from different organs of the halophyte Lycium europaeum L. Curr. Bioact. Compd. 2022, 18, 2. https://doi.org/10.2174/1573407217666210713101441.
- Mejri, H.; Ouerghemi, I.; Wannes, W.A.; Haddada, F.M.; Tlili, N.; Hammami, M.; Dussault, C.; Lancette, K.G.-L.; Pichette, A.; Legault, J.; et al. Phytochemical analysis, antioxidant, anticancer and anti-inflammatory activities of Lycium europaeum fruits. Int. J. Environ. Health Res. 2022. https://doi.org/10.1080/09603123.2022.2115469.
- Baghdadi, H.; El-Sayed, S.H.; Salem, G.; Metwally, A.; Salem, A.M. A comparative chemical study of Lycium species growing in Egypt. Alex. J. Pharm. Sci. 1988, 2, 73–76.
- Ewais, E.A.; Abd El-Maboud, M.M.; Elhaw, M.H.; Haggag, M.I. Phytochemical studies on Lycium schweinfurthii var. schweinfurthii (Solanaceae) and isolation of five flavonoids from leaves. J. Med. Plants Stud. 2016, 4, 288–300.
- Elbermawi, A.; Halim, A.F.; Mansour, E.-S.S.; Ahmad, K.F.; Ashour, A.; Amen, Y.; Shimizu, K. A new glucoside with a potent α-glucosidase inhibitory activity from Lycium schweinfurthii. Nat. Prod. Res. 2021, 35, 976–983.
- Elbermawi, A.; Halim, A.F.; Mansour, El-S.S.; Ahmad, K.F.; Elsbaey, M.; Ashour, A.; Amen, Y.; El-Gamil, M.M.; Tomofumi, M.; Shimizu, K. Lycium schweinfurthii: New secondary metabolites and their cytotoxic activities. Nat. Prod. Res. 2022, 36, 5134–5141. https://doi.org/10.1080/14786419.2021.1922902.
- Bedair, H.; Shaltout, K.; Ahmed, D.; El-Din, A.S.; El-Fahhar, R. Characterization of the wild trees and shrubs in the Egyptian flora. Egypt. J. Bot. 2020, 60, 147–168.
- Mamdouh, D.; Mahgoub, H.A.M.; Gabr, A.M.M.; Ewais, E.A.; Smetanska, I. Genetic stability, phenolic, flavonoid, ferulic acid contents, and antioxidant activity of micropropagated Lycium schweinfurthii plants. Plants 2021, 10, 2089. https://doi.org/10.3390/plants10102089.
- Mamdouh, D.; Smetanska, I. Optimization of callus and cell suspension cultures of Lycium schweinfurthii for improved production of phenolics, flavonoids, and antioxidant activity. Horticulturae, 2022, 8, 394. https://doi.org/10.3390/horticulturae8050394.
- Abdennacer, B.; Karim, M.; Yassine, M.; Nesrine, R.; Mouna, D.; Mohamed, B. Determination of phytochemicals and antioxidante acivity of mthanol extracts obtained from the fruit and leaves of Tunisian Lycium intricatum Boiss. Food Chem. 2015, 174, 577–584.
- Jiang, Y.; Fang, Z.; Leonard, W.; Zhang, P. Phenolic compounds in Lycium berry: Composition, health benefits and industrial applications. J. Funct. Foods 2021, 77, 104340. https://doi.org/10.1016/j.jff.2020.104340.
- Bendjedou, H.; Maggi, F.; Bennaceur, M.; Mancinelli, M.; Benamar, H.; Barboni, L. A new ionone derivative from Lycium intricatum Boiss. (Solanaceae). Nat. Prod. Res. 2022, 36, 687–694.
- Boulila, A.; Bejaoui, A. Lycium intricatum Boiss.: An unexploited and rich source of unsaturated fatty acids, 4-desmethylsterols and other valuable phytochemicals. Lipids Heath Dis. 2015, 14, 59. https://doi.org/10.1186/s12944-015-0055-9.
- Idm’hand, E.; Msanda, F.; Cherifi, K. Ethnobotanical study and biodiversity of medicinal plants used in the Tarfaya Province, Morocco. Acta Ecol. Sin. 2020, 40, 134–144.
- Fatiha, B.A.; Souad, S.; Ouafe, B.; Jamila, D.; Allal, D.; Lahcen, Z. Ehnobotanical study of medicinal plants used in the region of Middle Oumrbia (Morocco). Plant Arch. 2019, 19, 2005–2017.
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Berries: Anti-inflammatory effects in humans. J. Agric. Food Chem. 2014, 62, 3886–3903.
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66.
- Zhang, D.; Xia, T.; Dang, S.; Fan, G.; Wang, Z. Investigation of Chinese wolberry (Lycium spp.) germplasm by restriction site- associated DNA sequencing (RAD-seq). Biochem. Genet. 2018, 56, 575–585.
- Fukuda, T.; Yokoyama, J.; Ohashi, H. Phylogeny and biogeography of the genus Lycium (Solanaceae): Inferences from chloroplast DNA sequences. Mol. Phylogenet. Evol. 2001, 19, 246–258.
- Zhou, Z.; Xiao, J.; Fan, H.; Yu, Y.; He, R.; Feng, X.; Gao, H. Polyphenols from wolfberry and their bioactivities. Food Chem. 2017, 214, 644–654.
- Xing, L.; Wang, Y.; Luo, R.; Li, X.; Zou, L. Determination of 31 pesticide residues in wolfberry by LC-MS/MS and dietary risk assessment of wolfberry consumption. Food Sci. Tecnol. 2022, 42, e61921. https://doi.org/10.1590/fst.61921.
- Kulczyński, B.; Gramza-Michałowska, A. Gojy berry (Lycium barbarum): Composition and health effects—A review. Pol. J. Food Nutr. Sci. 2016, 66, 67–76.
- Liang, Z.; Luo, Z.; Li, W.; Yang, M.; Wang, L.; Lin, X.; Li, L. Elevated CO2 enhanced the antioxidant activity and downregulated cell wall metabolism of wolfberry (Lycium barbarum L.). Antioxidants 2022, 11, 16. https://doi.org/10.3390/antiox11010016.
- Meng, J.; Liu, Z.; Gou, C.L.; Rogers, K.M.; Yu, W.-J.; Zhang, S.-S.; Yuan, Y.-W.; Zhang, L. Geographical origin of Chinese wolfberry (goji) determined by carbon isotope analysis of specific volatile compounds. J. Chromatogr. B 2019, 1105, 104–112.
- Yin, T.; Guo, T.; Ma, Z.; Wang, Z.; Sun, X.; Li, C. Classification of wolfberry with different geographical origins by using voltametric electronic tongue. IFAC PapersOnLine 2018, 51, 654–659.
- Yang, Z.; Wang, Z.; Yuan, W.; Li, C.; Jing, X.; Han, H. Classification of wolfberry from different geographical origins by using electronic tongue and deep learning algorithm. IFAC Papers OnLine 2019, 52, 397–402.
- Zhang, S.; Wei, Y.; Wei, S.; Liu, H.; Guo, B. Authentication of Zhongning wolfberry with geographical indication by mineral profile. Int. J. Food Sci. Technol. 2017, 52, 457–463.
- Dong, F.; Hao, J.; Luo, R.; Zhang, Z.; Wang, S.; Wu, K. Identification of the proximate geographical origin of wolfberries by two-dimensional correlation spectroscopy combined with deep learning. Comput. Electron. Agric. 2022, 198, 107027. https://doi.org/10.1016/j.compag.2022.107027.
- Du, M.; Gong, Y.; Lin, Z.Z.; Shi, X.Y.; Hua, G.D.; Qiao, Y.J. Rapid identification of wolfberry fruit of different geographic regions with sample surface near infrared spectra combined with multi-class SVM. Spectrosc. Spect. Anal. 2013, 33, 1211–1214.
- Zhong, L.; Liu, M.D.; Ji, S.X. The identification of the origin of Chinese wolfberry based on infrared spectral technology and the artificial neural network. Spectrosc. Spect. Anal. 2016, 36, 720–723.
- Gong, H.; Rehman, F.; Li, Z.; Liu, J.; Yang, T.; Liu, J.; Li, H.; Hu, Z.; Ma, Q.; Wu, Z.; et al. Discrimination of geographical origins of wolfberry (Lycium barbarum L.) fruits using stable isotopes, earth elements, free amino acids, and saccharides. J. Agric. Food Chem. 2022, 70, 2984–2997.
- Bondia-Pons, I.; Savolainen, O.; Törrönen, R.; Martinez, J.A.; Poutanen, K.; Hanhineva, K. Metabolic profiling of Gogi berry extracts for discrimination of geographical origin by non-targeted liquid chromatography couples to quadrupole time-of-flight mass spectrometry. Food Res. Int. 2014, 63, 132–138.
- Zhang, X.; Zhao, Z.; Wei, S.; Du, G.; Ji, Q.; Zhang, L.; Wei, H. Study on the design and control system for wolfberry harvesting robot. In Proceedings of the 28th Chinese Control and Decision Conference, Yinchuan, China, 28–30 May 2016; pp. 5984–5985.
- Wang, J.; Mei, S.; Xiao, G.; Zhou, H. Research on mechanized harvesting methods of Lycium barbarum fruit. IFAC papersOnLine 2018, 51, 223–226.
- Chen, Y.; Zhao, J.; Hu, G.; Chen, J. Design and testing of a pneumatic oscillating Chinese wolfberry harvester. Horticulturae 2021, 7, 214. https://doi.org/10.3390/horticulturae7080214.
- Ban, Z.; Wei, W.; Yang, X.; Feng, J.; Guan, J.; Li, L. Combination of heat treatment and chitosan coating to improve postharvest quality of wolfberry (Lycium barbarum). Int. J. Food Sci. Technol. 2015, 50, 1019–1025.
- Xiang, W.; Wang, H.-W.; Tian, Y.; Sun, D.-W. Effects of salicylic acid combined with gas atmospheric control on postharvest quality and storage stability of wolfberries: Quality attributes and interaction evaluation. J. Food Process Eng. 2021, 44, e13764. https://doi.org/10.1111/jfpe.13764.
- .Ling, L.; Zhao, Y.; Tu, Y.; Yang, C.; Ma, W.; Feng, S.; Lu, L.; Zhang, J. The inhibitory effect of volatile organic compounds produced by Bacillus subtilis CL2 on pathogenic fungi of wolfberry. J. Basic Microbiol. 2021, 61, 110–121.
- Yang, M.; Ding, C.; Zhu, J. The drying quality and energy consumption of Chinese wolfberry fruits under electrohydrodynamic system. Int. J. Appl. Electromagn. Mech. 2017, 55, 101–112.
- Yang, M.; Ding, C. Electrohydrodynamic (EHD) drying of the Chinese wolfberry fruits. SpringerPlus 2016, 5, 909. https://doi.org/10.1186/s40064-016-2546-1.
- Ni, J.; Ding, C.; Zhang, Y.; Song, Z.; Hu, X.; Hao, T. Effect of electrohydrodynamic partially combined with oven drying on Chinese wolfberry. Int. J. Appl. Electromagn. Mech. 2020, 63, 465–482.
- Ni, J.; Ding, C.; Zhang, Y.; Song, Z.; Hu, X.; Hao, T. Electrohydrodynamic drying of Chinese wolfberry in a multiple needle-to-plate electrode system. Foods, 2019, 8, 152. https://doi.org/10.3390/foods8050152.
- Xie, L.; Zengh, Z.-A.; Mujumdar, A.S.; Fang, X.-M.; Wang, J.; Zhang, Q.; Ma, Q.; Xiao, H.-W.; Liu, Y.-H.; Gao, Z.-J. Pulsed vacum drying (PVD) of wolfberry: Drying kinetics and quality attributes. Drying Technol. 2018, 36, 1501–1514.
- Yu, F.; Li, Y.; Wu, Z.; Wang, X.; Wan, N.; Yang, M. Dehydration of wolfberry fruit using pulsed vacuum drying combined with carboxymethyl celulose coating pretreatment. LWT–Food Sci. Technol. 2020, 134, 110159. https://doi.org/10.1016/j.lwt.2020.110159.
- Hu, Z.; Zhang, S.; Chu, W.; He, W.; Yu, C.; Yu, H. Numerical analysis and preliminar experiment of a solar assisted heat pump drying system for Chinese wolfberry. Energies 2020, 13, 4306. https://doi.org/10.3390/en13174306.
- Xie, L.; Mujumdar, A.S.; Fang, X.-M.; Wang, J.; Dai, J.-W.; Du, Z.-L.; Xiao, H.-W.; Liu, Y.; Gao, Z.-J. Far-infrared radiation heating assisted pulsed vacuum drying (FIR-PVD) of wolfberry (Lycium barbarum L.): Effects on drying kinetics and quality attributes. Food Bioprod. Process. 2017, 102, 320–331.
- Qi, Y.; Yu, F.; Wang, X.; Wan, N.; Yang, M.; Wu, Z.; Li, Y. Drying of wolfberry fruit juice using low-intensity pulsed ultrasound. LWT–Food Sci. Technol. 2021, 141, 110953. https://doi.org/10.1016/j.lwt.2021.110953.
- Xu, Y.; Zang, Z.; Zhang, Q.; Wang, T.; Shang, J.; Huang, X.; Wan, F. Characteristics and quality analysis of radio frequency-hot air combined segmented drying of wolfberry (Lycium barbarum). Foods 2022, 11, 1645. https://doi.org/10.3390/foods11111645.
- Cui, C.; Zhao, D.; Huang, J.; Hao, J. Progress on research and development of goji berry drying: A review. Int. J. Food Propert. 2022, 25, 435–449.
- Fan, J.; Lin, L.; Zhao, M. Construction of in vitro fermentation model using gut microbiota relating to glucose and lipid metabolism: A supplementary method for initial screening of polysaccharides with hypoglycemic potentials. J. Sci. Food Agric. 2022, 102, 6328–6339. https://doi.org/10.1002/jsfa.11983.
- Ma, R.-H.; Zhang, X.-X.; Ni, Z.-J.; Thakur, K.; Wang, W.; Yan, Y.-M.; Cao, Y.-L.; Zhang, J.-G.; Rengasamy, K.R.R.; Wei, Z.-J. Lycium barbarum (goji) as functional food: A review of its nutrition, phytochemical structure, biological features, and food industry prospects. Crit. Rev. Food Sci. Nutr. 2022. https://doi.org/10.1080/10408398.2022.2078788.
- Wang, Y.; Jin, H.; Dong, X.; Yang, S.; Ma, S.; Ni, J. Quality evaluation of Lycium barbarum (wolfberry) from different regions in China based on polysaccharide structure, yield and bioactivies. Chin. Med. 2019, 14, 49. https://doi.org/10.1186/s13020-019-0273-6.
- Potterat, O. Goji (Lycium barbarum and L. L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7–19.
- Ma, R.-H.; Zhang, X.-X.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. Research progress of Lycium barbarum L. as functional food: Phytochemical composition and health benefits. Curr. Opin. Food Sci. 2022, 47, 100871. https://doi.org/10.1016/j.cofs.2022.100871.
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium barbarum: A traditional Chinese herb and a promising anti-aging agent. Aging Dis. 2017, 8, 778–791.
- Chen, D.; Guo, S.; Zhou, J.; Zhu, Y.; Zhang, F.; Zeng, F.; Duan, R.; Xu, M.; Duan, J.-A. Chemical constituents from Lycium barbarum (Solanaceae) and their chemophenetic significance. Bioch. Syst. Ecol. 2021, 97, 104292. https://doi.org/10.1016/j.bse.2021.104292.
- Liu, J.; Gong, Y.; Yang, J.; Di, D. Advance on alkaloids of Lycium genus. Chin. Sci. Bull. 2022, 67, 332–350.
- Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Dijuriš, J.D.; Ilić, T.D.; Kostić, A.Z.; Pešić, M.B. Health benefits and applications of goji berries in functional food products development: A review. Antioxidants 2022, 11, 248. https://doi.org/10.3390/antiox11020248.
- Liu, S.Y.; Wang, Q.Q.; Lei, Y.H.; Wang, S.S.; Chen, K.L.; Li, Y.; Xiong, J.; Liang, X.J.; Zhou, X.; Li, Y.K. Elucidating the interaction of rhizosphere bacteria and environmental factors in influencing active ingredient content of Lycium barbarum fruit in China. J. Appl. Microbiol. 2022, 132, 3783–3796.
- Sim, R.H.; Sirasanagandla, S.R.; Das, S.; Teoh, S.L. Treatment of glaucoma with natural products and their mechanism of action: An update. Nutrients 2022, 14, 534. https://doi.org/10.3390/nu14030534.
- Rang, Y.; Liu, H.; Liu, C. Potential for non-starch polysaccharides in the prevention and remediation of cognitive impairment: A comprehensive review. Int. J. Biol. Macromol. 2022, 208, 182–195.
- Qi, Y.; Duan, G.; Fan, G.; Peng, N. Effect of Lycium barbarum polysaccharides on cell signal transduction pathways. Biomed. Pharmacother. 2022, 147, 112620. https://doi.org/10.1016/j.biopha.2022.112620.
- Xiao, Z.; Deng, Q.; Zhou, W.; Zhang, Y. Immune activities of polysaccharides isolated from Lycium barbarum L.. What do we know so far? Pharmacol. Therap. 2022, 229, 107921. https://doi.org/10.1016/j.pharmthera.2021.107921.
- Huang, R.; Wu, E.; Deng, X. Potential of Lycium barbarum polysaccharide for the control of glucose and lipid metabolism disroders: A review. Int. J. Food Prop. 2022, 25, 673–680.
- Pinilla, I.; Maneu, V.; Campello, L.; Fernández-Sánchez, L.; Martínez-Gil, N.; Kutsyr, O.; Sánchez-Sáez, X.; Sánchez-Castillo, C.; Lax, P.; Cuenca, N. Inherited retinal dystrophies: Role of oxidative stress and inflammation in their physiopathology and therapeutic implications. Antioxidants 2022, 11, 1086. doi.org/10.3390/antiox11061086.
- Yang, C.; Zhao, Q.; Li, S.; Pu, L.; Yu, L.; Liu, Y.; Lai, X. Effects of Lycium barbarum L: Polysaccharides on vascular retinopathy: An insight review. Molecules 2022, 27, 5628. doi.org/10.3390/molecules27175628.
- Liu, H.; Cui, B.; Zhang, Z. Mechanism of glycometabolism regulation by bioactive compounds from the fruits of Lycium barbarum: A review. Food Res. Int. 2022, 159, 111408. doi.org/10.1016/j.foodres.2022.111408.
- Rohilla, P.; Jain, H.; Chhikara, A.; Singh, L.; Dahiya, P. Anticancer potential of Solanaceae plants: A review. S. Afr. J. Bot. 2022, 149, 269–289.
- Zhang, Z.; Wang, S.; Tan, H.; Yang, P.; Li, Y.; Xu, L.; Duan, B.; Liu, Y. Advances in polysaccharides of natural source of the anti-Alzheimer’s disease effect and mechanism. Carbohydr. Polym. 2022, 296, 119961. doi.org/10.1016/j.carbpol.2022.119961.
- Xu, W.; Jiang, Y.; Wang, N.; Bai, H.; Xu, S. Traditional Chinese medicine as a promising strategy for the treatment od Alzheimer’s disease complicated with osteoporosis. Front. Pharmacol. 2022, 13, 842101. https://doi.org/10.3389/fphar.2022.842101.
- Liu, Y.; Li, C.; Shen, X.; Liu, Y. The use of traditional Chinese medicines in relieving exercise-induced fatigue. Front. Pharmacol. 2022, 13, 969827. https://doi.org/10.3389/fphar.2022.969827.
- Che, Z.; Zhou, Z.; Xiao, J. Mechanisms of wolfberry in the treatment of liver diseases. Chin. Sci. Bull. 2022, 67, 351–363.
- Ren, Y.; Li, S.; Song, Z.; Luo, Q.; Zhang, Y.; Wang, H. The regulatory roles of polysaccharides and ferroptosis-related phytochemicals in liver diseases. Nutrients 2022, 14, 2303. https://doi.org/10.3390/nu14112303.
- Wang, Y.; Chen, R.; Yang, Z.; Wen, Q.; Cao, X.; Zhao, N.; Yan, J. Protective effects of polysaccharides in neurodegenerative diseases. Front. Aging Neurosci. 2022, 14, 917629. https://doi.org/10.3389/fnagi.2022.917629.
- Feng, Y.; Song, Y.; Zhou, J.; Duan, Y.; Kong, T.; Ma, H.; Zhang, H. Recent progress of Lycium barbarum polysaccharides on intestinal microbiota, microbial metabolites and health: A review. Crit. Rev. Food Sci. Nutr. 2022, https://doi.org/10.1080/10408398.2022.2128037.
- Cao, C.; Wang, Z.; Gong, G.; Huang, W.; Huang, L.; Song, S.; Zhu, B. Effects of Lycium barbarum polysaccharides on immunity and metabolic syndrome associated with the modulation of gut microbiota: A review. Foods 2022, 11, 3177. doi.org/10.3390/foods11203177.
- Wang, B.; Han, L.; Liu, J.-M.; Zhang, J.; Wang, W.; Li, B.-G.; Dong, C.-X.; Bai, C.-C. Lycium genus polysaccharides: An overview of its extraction, structures, pharmacological activities and biological applications. Separations 2022, 9, 197. https://doi.org/10.3390/separations9080197.
- Gong, H.; Li, W.; Sun, J.; Guan, Q.; Guo, Y.; Wang, Y. A review on plant polysaccharide based on drug delivery system for construction and application, with emphasis on traditional Chinese medicine polysaccharide. Int. J. Biol. Macromol. 2022, 211, 711–728.
- Wannes, W.A.; Tounsi, M.S. Phytochemical composition and health properties of Lycium europaeum L.: A review. Acta Ecol. Sinica 2021, 41, 390–401.
- Yao, X.; Peng, Y.; Xu, L.-J.; Li, L.; Wu, Q.-L.; Xiao, P.-G. Phytochemical and biological studies of Lycium medicinal plants. Chem. Biodivers. 2011, 8, 976–1010.
- Akter, F.; Rahman, M.S.; Al Amin, G.M.; Miah, M.I.; Koh, Y.-S. Beneficial therapy with natural anti-inflammatory agents and supplements. J. Bacteriol. Virol. 2021, 51, 149–162.
- Ikanya, L.W.; Maina, J.G.; Gachuiri, C.K.; Owino, W.O.; Dubeux, J.C.B., Jr. Chemical composition, and digestibility of preferred forage species by lactating Somali camels in Kenya. Rangel. Ecol. Manag. 2022, 80, 61–67.
- Chaachouay, N.; Azeroual, A.; Bencharki, B.; Douira, A.; Zidane, L. Ethnoveterinary medicinal plants for animal therapy in the Rif, North of Morocco. S. Afr. J. Bot. 2022, 147, 176–191.
- Mollel, N.P.; Otieno, J.N.; Sitoni, D.K. Medicinal plants traded in Arusha city, Tanzania. J. Med. Plants Stud. 2022, 10, 175–182.
- El-Mokasabi, F. Survey of wild trees and shrubs in Eastern region of Libya and their economical value. Alq. J. Med. App. Sci. 2022, 5, 48–55.
- Almushghub, F.; Ahmed, D.; El-Din, A.S.; Shaltout, K. Vegetation analysis of Wadi Kaam at northwest Libya. J. Basic Environm. Sci. 2022, 9, 20–37.
- Bendjedou, H.; Bennaceura, M.; Benamar, H.; Rodrigues, M.J.; Pereira, C.; Bensouicie, C.; Custódio, L. In vitro antioxidant and enzyme inhibitory properties and phenolic contents of crude extracts and fractions from different organs of the halophyte Lycium europaeum L. Curr. Bioact. Compd. 2022, 18, 2. https://doi.org/10.2174/1573407217666210713101441.
- Mejri, H.; Ouerghemi, I.; Wannes, W.A.; Haddada, F.M.; Tlili, N.; Hammami, M.; Dussault, C.; Lancette, K.G.-L.; Pichette, A.; Legault, J.; et al. Phytochemical analysis, antioxidant, anticancer and anti-inflammatory activities of Lycium europaeum fruits. Int. J. Environ. Health Res. 2022. https://doi.org/10.1080/09603123.2022.2115469.
- Khodaparast, S.A.; Darsaraei, H.; Abbasi, M. The genus Arthrocladiella: A new report of powdery mildew fungi from Iran. Mycol. Iran. 2021, 8, 135–140.
- Ajjoun, M.; Kharchoufa, L.; Merrouni, I.A.; Elachouri, M. Moroccan medicinal plants traditionally used for the treatment of skin diseases: From ethnobotany to clinical trials. J. Ethnopharmacol. 2022, 297, 115532. https://doi.org/10.1016/j.jep.2022.115532.
- Jaadan, H.; Akodad, M.; Moumen, A.; Baghour, M.; Skalli, A.; Ezrari, S.; Belmalha, S. Ethnobotanical survey of medicinal plants growing in the region of “Oulad Daoud Zkhanine” (Nador Province), in Northeastern Morocco. Ethnobot. Res. Appl. 2020, 19, 39. http://dx.doi.org/10.32859/era.19.39.1-12.
- Montoleone, E.; Longo, D.; Cassanego, E.; Lazzeri, V. Lycium schweinfurthii Dammer (Solanaceae, Lycieae), species mal compressa confermata per meridione d’Italia. Acta Plant. Notes 2022, 8, 11–16.
- Jamous, R.M.; Zaitoun, S.Y.A.; Husein, A.I.; Qasem, I.B.Y.; Ali-Shtayeh, M.S. Screening for biological activities of medicinal plants used in traditional Arabic Palestinian herbal medicine. Eur. J. Med. Plants 2015, 9, 1–13.
- Auda, M.A. An ethnobotanical uses of plants in the Middle Area, Gaza Strip, Palestine. Adv. Environ. Biol. 2011, 5, 3681–3687.
- Ighbareyeh, J.M.H.; Cano-Ortiz, A.; Cano, E. Phytosociology and vegetation of plants of Beit Jibrin in Palestine. Land 2022, 11, 264. https://doi.org/10.3390/land11020264.
- Baghdadi, H.; El-Sayed, S.H.; Salem, G.; Metwally, A.; Salem, A.M. A comparative chemical study of Lycium species growing in Egypt. Alex. J. Pharm. Sci. 1988, 2, 73–76.
- Ewais, E.A.; Abd El-Maboud, M.M.; Elhaw, M.H.; Haggag, M.I. Phytochemical studies on Lycium schweinfurthii var. schweinfurthii (Solanaceae) and isolation of five flavonoids from leaves. J. Med. Plants Stud. 2016, 4, 288–300.
- Elbermawi, A.; Halim, A.F.; Mansour, E.-S.S.; Ahmad, K.F.; Ashour, A.; Amen, Y.; Shimizu, K. A new glucoside with a potent α-glucosidase inhibitory activity from Lycium schweinfurthii. Nat. Prod. Res. 2021, 35, 976–983.
- Elbermawi, A.; Halim, A.F.; Mansour, El-S.S.; Ahmad, K.F.; Elsbaey, M.; Ashour, A.; Amen, Y.; El-Gamil, M.M.; Tomofumi, M.; Shimizu, K. Lycium schweinfurthii: New secondary metabolites and their cytotoxic activities. Nat. Prod. Res. 2022, 36, 5134–5141. https://doi.org/10.1080/14786419.2021.1922902.
- Bedair, H.; Shaltout, K.; Ahmed, D.; El-Din, A.S.; El-Fahhar, R. Characterization of the wild trees and shrubs in the Egyptian flora. Egypt. J. Bot. 2020, 60, 147–168.
- Mamdouh, D.; Mahgoub, H.A.M.; Gabr, A.M.M.; Ewais, E.A.; Smetanska, I. Genetic stability, phenolic, flavonoid, ferulic acid contents, and antioxidant activity of micropropagated Lycium schweinfurthii plants. Plants 2021, 10, 2089. https://doi.org/10.3390/plants10102089.
- Wu, T.; Kerbler, S.M.; Fernie, A.R.; Zhang, Y. Plant cell cultures as heterologous bio-factories for secondary metabolite production. Plant Comm. 2021, 2, 100235.
- Mamdouh, D.; Smetanska, I. Optimization of callus and cell suspension cultures of Lycium schweinfurthii for improved production of phenolics, flavonoids, and antioxidant activity. Horticulturae, 2022, 8, 394. https://doi.org/10.3390/horticulturae8050394.
- Elbermawi, A.; Ali, A.R.; Amen, Y.; Ashour, A.; Ahmad, K.F.; Mansour, E.-S.S.; Halim, A.F. Anti-diabetic activities of phenolic compounds of Alternaria sp., an endophyte isolated from the leaves of desert plants growing in Egypt. RSC Adv. 2022, 12, 24935. https://doi.org/10.1039/d2ra02532a.
- Chen, A.; Mao, X.; Sun, Q.; Wei, Z.; Li, J.; You, Y.; Zhao, J.; Jiang, G.; Wu, Y.; Wang, L.; et al. Alternaria mycotoxins: An overview of toxicity, metabolism, and analysis in food. J. Agric. Food Chem. 2021, 69, 7817–7830.
- Lombardo, E.; Bancheva, S.; Domina, G.; Venturella, G. Distribution, ecological role and symbioses of selected shrubby species in the Mediterranean basin: A review. Plant Biosyst. 2020, 154, 438–454.
- Obón, C.; Rivera, D.; Verde, A.; Alcaraz, F. Ethnopharmacology and medicinal uses of extreme halophytes. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020; pp. 1–27. https://doi.org/10.1007/978-3-030-17854-3_107-1.
- Marrero-Rodríguez, N.; García-Romero, L.; Hrnández-Cordero, A.I.; Peña-Alonso, C.; Pérez-Chacón Espino, E. Deforestation by historical lime industry in an arid aeolian sedimentary system: An applied and methodological research. Sci. Total Environ. 2022, 819, 152009. http://dx.doi.org/10.1016/j.scitotenv.2021.152009.
- Midhat, L.; Ouazzani, N.; Hejjaj, A.; Ouhammou, A.; Mandi, L. Accumulation of heavy metals in metallophytes from three mining sites (Southern Centre Morocco) and evaluation of their phytoremediation potential. Eotoxicol. Environ. Saf. 2019, 169, 150–160.
- Idm’hand, E.; Msanda, F.; Cherifi, K. Ethnobotanical study and biodiversity of medicinal plants used in the Tarfaya Province, Morocco. Acta Ecol. Sin. 2020, 40, 134–144.
- Fatiha, B.A.; Souad, S.; Ouafe, B.; Jamila, D.; Allal, D.; Lahcen, Z. Ehnobotanical study of medicinal plants used in the region of Middle Oumrbia (Morocco). Plant Arch. 2019, 19, 2005–2017.
- Abdennacer, B.; Karim, M.; Yassine, M.; Nesrine, R.; Mouna, D.; Mohamed, B. Determination of phytochemicals and antioxidante acivity of mthanol extracts obtained from the fruit and leaves of Tunisian Lycium intricatum Boiss. Food Chem. 2015, 174, 577–584.
- Jiang, Y.; Fang, Z.; Leonard, W.; Zhang, P. Phenolic compounds in Lycium berry: Composition, health benefits and industrial applications. J. Funct. Foods 2021, 77, 104340. https://doi.org/10.1016/j.jff.2020.104340.
- Boulila, A.; Bejaoui, A. Lycium intricatum Boiss.: An unexploited and rich source of unsaturated fatty acids, 4-desmethylsterols and other valuable phytochemicals. Lipids Heath Dis. 2015, 14, 59. https://doi.org/10.1186/s12944-015-0055-9.
- Bendjedou, H.; Maggi, F.; Bennaceur, M.; Mancinelli, M.; Benamar, H.; Barboni, L. A new ionone derivative from Lycium intricatum Boiss. (Solanaceae). Nat. Prod. Res. 2022, 36, 687–694.
- Talukdar, R.; Paul, S.; Tayung, K. Antifungal drugs from endophytic microbes: Present and future prospects. In Bioresource utilization and management. Applications in Therapeutics, Biofuels, Agriculture, and Environmental Science; Thatoi, H.; Das, S.K.; Mohapatra, S., Eds.; Apple Acacemic Press: Palm Bay, FL, USA, 2022; pp. 3–37.
- Khattab, A.R.; Farag, M.A. Marine and terrestrial endophytic fungi: A mine of bioactive xanthone compounds, recent progress, limitations, and novel applications. Crit. Rev. Biotechnol. 2022, 42, 403–430.
- Zhang, W.; Krohn, K.; Egold, H.; Draeger, S.; Schulz, B. Diversity of antimicrobial pyrenophorol derivatives from an endophytic fungus, Phoma sp. Eur. J. Org. Chem. 2008, 4320-4328.
- Krohn, K.; Kouam, S.F.; Kuigoua, G.M.; Hussain, H.; Cludius-Brandt, S.; Flörke, U.; Kurtán, T.; Pescitelli, G.; di Bari, L.; Draeger, S.; Schulz, B. Xanthones and oxepino[2,3-b]chromones from three endophytic fungi. Chem. Eur. J. 2009, 15, 12121-12132.
- Siddiqui, I.N.; Zahoor, A.; Hussain, H.; Ahmed, I.; Ahmad, V.U.; Padula, D.; Draeger, S.; Schulz, B.; Meier, K.; Steiner, M.; Kurtán, T.; Flörke, U.; Pescitelli, G.; Krohn, K. Diversonol and blennolide derivatives from the endophytic fungus Microdiplodia sp.: absolute configuration of diversonol. J. Nat. Prod. 2011, 74, 365-373.
- Sharma, P.; Singh, S.P. Role of the endogenous fungal metabolites in the plant growth improvement and stress. Fungi Bio-prospects in Sustainable Agriculture, Environment and Nano-technology 2021, 381-401. DOI: https://doi.org/10.1016/B978-0-12-821734-4.00002-2.
- Qian, D.; Zhao, Y.; Yang, G.; Huang, L. Systematic review of chemical constituents in the genus Lycium (Solanaceae). Molecules 2017, 22, 911. https://doi.org/10.3390/molecules22060911.




