Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Jinsol Han and Version 3 by Camila Xu.
Alcoholic liver disease (ALD) is a globally prevalent chronic liver disease caused by chronic or binge consumption of alcohol. However, the therapeutic efficiency of current therapies for ALD is limited, and there is no FDA-approved therapy for ALD at present. MRecently, mesenchymal stem cells (MSCs) have emerged as a promising candidate for ALD treatment and have been tested in several clinical trials. MSC-released factors have captured attention, as they have the same therapeutic function as MSCs.
- alcoholic liver disease
- therapeutics
- mesenchymal stem cells
1. Stem Cell Therapy for Alcoholic Liver Disease (ALD)
Despite the promising results of therapeutic candidates for ALD in preclinical studies, their efficacy has been less than expected or not observed in clinical trials. The therapeutic effects of stem cells have been proved in chronic liver diseases such as ALD, NAFLD and acute liver failure [1][2][176,177]. Pluripotent stem cells, embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) can differentiate into hepatocyte-like cells [3][178]. The transplantation of hepatocyte-like cells derived from human ESCs alleviated CCl4-induced liver damage by replacing damaged cells and promoting liver regeneration compared to the control group receiving cell medium without ESCs [4][179]. Transplantation of iPSCs-derived hepatocyte-like cells also improved survival rate of mice with acute liver failure [5][180]. Although they do not have the ability to differentiate as much as ESCs and iPSCs, MSCs are also multipotent [1][6][176,181]. In addition, they have low immunogenicity [1][6][176,181]. Hence, MSCs are a commonly used and widely studied in stem cell therapy for liver diseases [1][6][176,181]. In high fat diet-induced NAFLD mice, MSC transplantation significantly reduced inflammation and steatosis by suppressing the activation of CD4+ T cells or rescuing mitochondria dysfunction [7][8][182,183]. Recently, comprehensive studies have been conducted on the therapeutic effects of EVs on liver diseases [9][184]. Hence, MSC-based therapies emerge as an attractive treatment option for ALD [10][11][12][21,185,186]. An understanding of the protective effects of MSCs and the underlying mechanisms is needed to develop safe and effective MSC-based therapeutic agents for ALD [11][12][13][185,186,187]. In this section, reswearchers review the effects of MSCs and MSC-derived factors on ALD progression (Figure 12).
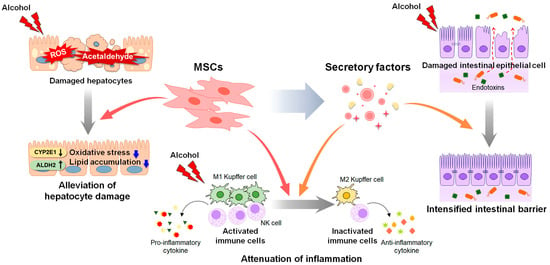
Figure 12. Therapeutic actions of MSCs and MSC-derived secretory factors in ALD. Mesenchymal stem cells (MSCs) and MSC-derived secretome have therapeutic potential for alcohol-induced liver damage. MSC transplantation decreases the expression of cytochrome P450 2E1 (CYP2E1) and increases the activity of the acetaldehyde-metabolizing enzyme aldehyde dehydrogenase 2 (ALDH2), reducing the levels of reactive oxygen species (ROS) and acetaldehyde induced by alcohol. Decreased ROS and acetaldehyde lower oxidative stress and lipid accumulation in hepatocytes. MSCs exert anti-inflammatory property by suppressing the activation of immune cells, including Kupffer cells and natural killer (NK) cells, and the secretion of pro-inflammatory cytokines. MSC-derived secretome such as cytokines, chemokines, free nucleic acids, and extracellular vesicles, alleviates hepatic inflammation by promoting polarization of Kupffer cells toward anti-inflammatory M2 phenotype. In addition, MSC-derived secretome directly improves viability of alcohol-damaged intestinal epithelial cells, and intensifies the intestinal barrier, blocking leakage of endotoxins from intestine.
2. Direct Transplantation of MSCs in ALD Treatment
Stem cells, including MSCs, have been directly transplanted, and their successful repair effects in various diseases have been proven [13][14][15][187,188,189]. Accumulating evidence has shown the therapeutic functions of MSCs originating from various sources in liver disease, including ALD [16][17][18][19][22,26,30,190]. Transplantation of BM-derived MSCs has been shown to significantly alleviate alcohol-caused liver damage, such as lipid accumulation, oxidative stress, and inflammation, in mice with AH [20][191]. Ge et al. [21][192] showed that BM-MSC transplantation reduced the activation of NK B cells and the secretion of IL-18, a pro-inflammatory cytokine in alcohol-fed mice. Transplantation of human adipose-derived MSCs effectively decreased CYP2E1 expression and increased the activity of the acetaldehyde-metabolizing enzyme ALDH2, alleviating alcohol-induced damage, including lipid accumulation and fibrosis [22][193]. Based on findings obtained from preclinical studies, MSCs have been administered to patients with alcoholic cirrhosis [23][194]. In several clinical trials, BM-MSCs administered via intravenous injection significantly improved liver histology and Child–Pugh scores indicating better liver function [24][25][26][195,196,197]. Furthermore, the expression of fibrosis-related markers, such as TGF-β1, collagen type 1, and α-smooth muscle actin, was significantly downregulated in patients after BM-MSC treatment [24][25][26][195,196,197]. However, several hurdles remain to be overcome before BM-MSCs can be approved for clinical applications [27][198]. Despite the successful outcomes of clinical trials, some researchers have cast doubt on the therapeutic effects of MSC transplantation in patients with alcoholic cirrhosis [28][29][199,200]. Rajaram et al. [28][199] showed that the clinical benefits of MSC transplantation were maintained in patients with advanced cirrhosis for only 8 weeks after MSC transplantation. Spahr et al. [29][200] reported that BM-MSC transplantation resulted in little improvement in liver histology and function among patients with alcoholic cirrhosis compared with standard medical therapy. In addition, further studies, including large-scale clinical trials, are required to verify the effectiveness of the long-term clinical application of MSCs.
3. Potential of Cell-Free Strategies for ALD Treatment
MSCs secrete a variety of factors, including cytokines, chemokines, free nucleic acids, and extracellular vesicles (EVs), in response to physiological or pathological stimuli [30][201]. These MSC-derived secretomes and EVs share many characteristics with their origin, MSCs [31][202]. They mimic the therapeutic functions of MSCs, including the modulation of immune pathways, cell proliferation, and migration, leading to the creation of a regeneration-favorable microenvironment [31][202]. The protective role of tumor necrosis factor-inducible gene 6 protein (TSG-6), an anti-inflammatory cytokine released by MSCs, in the liver against NAFLD and fibrosis progression has been proven [32][33][34][203,204,205]. In a recent study, TSG-6 was shown to alleviate the levels of hepatic lipids, MDA and pro-inflammatory cytokines and elevate the amounts of GSH and anti-inflammatory cytokines in TSG-6-treated mice with AH [35][206]. In this experimental animal model, TSG-6 induced the polarization of Kupffer cells toward an M2 phenotype, and reduced hepatic inflammation and STAT3 activation [35][36][206,207]. HGF secreted from skeletal muscle satellite cell-derived MSCs (skMSCs) significantly ameliorated alcohol-induced liver damage in binge alcohol-fed mice [37][208]. HGF from skMSCs directly recovered the viability and permeability of ethanol-exposed intestinal epithelial cells and intensified the intestinal barrier to suppress hepatic inflammation induced by the leakage of gut-derived hepatotoxins [37][208]. However, studies on the therapeutic effect of stem cell-derived factors on ALD are limited because no animal models fully mimic the spectrum of human ALD [38][39][8,209]. Unlike humans, rodents have a natural aversion to alcohol and a much faster alcohol-catabolizing rate [40][210]. As a result, alcohol-induced liver pathology is different in rodent models of ALD and patients with ALD [40][210]. In particular, neutrophil infiltration is hardly detected in rodents during ALD pathogenesis, whereas it is one of the key features of alcoholic steatohepatitis in humans [41][211].
The therapeutic potential of stem cell-derived factors has been proven in other models of liver disease that share a common pathology with ALD [18][42][43][27,30,212]. These findings point to their therapeutic potential for ALD. For example, human UC-MSC-derived exosomes have been shown to reduce oxidative stress and inhibit apoptosis in mice with CCl4-induced liver failure [44][213]. Furthermore, glutathione peroxidase 1 in EVs derived from human UC-MSCs has been shown to play a key role in the recovery of hepatic oxidant injury and the reversal of oxidative stress-induced apoptosis by inducing extracellular signal-regulated protein kinase 1/2 phosphorylation and Bcl-2 expression [45][214]. MSC-derived exosomes have been found to improve liver regeneration [46][215]. EVs released from a human embryonic stem cell line, HuES9-derived MSCs, promoted liver regeneration processes by upregulating priming-phase genes, including proliferating cell nuclear antigen and cyclin D1, in a CCl4-induced liver injury model [47][216]. EVs from placenta-derived MSCs (PD-MSCs) ameliorated hepatic failure caused by bile duct ligation [48][217]. Furthermore, C-reactive protein in exosomes secreted by PD-MSCs triggered activation of the Wnt signaling pathway and upregulated vascular endothelial growth factor (VEGF) and VEGF receptor 2, which are involved in angiogenesis and liver regeneration [48][217].
MSC-derived factors have been widely studied in several liver diseases, including NAFLD, acute liver failure, and liver fibrosis, with studies focusing on their immunomodulatory effects [42][49][50][27,218,219]. Numerous studies have revealed that MSC-derived exosomes lower inflammation by reducing inflammatory cytokines or promoting M2 polarization of macrophages [50][51][52][219,220,221]. In a mouse model of acute liver failure, EVs all secreted from either adipose-MSCs or UC-MSCs downregulated inflammatory cytokines, such as IL-6, IL-1β, and TNF-α [53][222]. The administration of BM-MSC-EVs switched Kupffer cells to an anti-inflammatory phenotype by delivering IL-10 loaded in EVs to target Kupffer cells in mice with hepatic injury induced by hemorrhagic shock [54][223]. TSG-6 induced the trans-differentiation of activated HSCs into stem-like cells and alleviated liver fibrosis and regenerated hepatic function and structure [34][205]. Milk fat globule-epidermal growth factor 8 protein inhibited TGF-β signaling by decreasing the expression of TGF-β receptor 1 in HSCs and reducing extracellular matrix deposition and liver fibrosis in CCl4-injected mice [55][224]. In addition, MSC-EVs carrying microRNAs (miR), such as miR-486-5p, miR-150-5p and miR-125b, reduced liver fibrosis by inactivating HSCs [18][30]. Thus, accumulating data show that the secretome and EVs derived from MSCs mediate therapeutic effects of various types of liver disease, and imply that they have therapeutic potential in ALD treatment. Further investigations are necessary to obtain additional data on characteristics and action mechanism of MSC-released factors to support their therapeutic potential.
