Cardiothoracic surgical critical care medicine (CT-CCM) is a medical discipline centered on the perioperative care of diverse groups of patients. With an aging demographic and an increase in burden of chronic diseases the utilization of cardiothoracic surgical critical care units is likely to escalate. Given these projections, it is important to assess the state of cardiothoracic surgical intensive care, to develop goals and objectives for the future, and to identify knowledge gaps in need of scientific inquiry.
- cardiothoracic surgery
- intensive care medicine
- anesthesiology
- delirium
- acute kidney injury after cardiac surgery
1. Introduction
2. Part 1—The Current State of Cardiothoracic Surgical Critical Care Medicine as a Medical Science
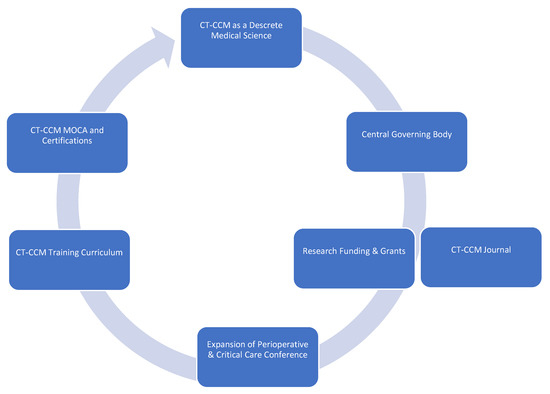
CT-CCM is a multidisciplinary endeavor composed of cardiothoracic surgeons, anesthesiologists, internists, pharmacists, perfusionists, and many more advanced level providers, nurses, and technicians (Figure 1) [2][16][2,16]. As such, the research pertaining to the CT-CCM patient populations is dispersed over many different professional societies and published in a variety of specialized journals. These include the American Thoracic Society, The Society of Thoracic Surgeons, Society of Critical Care Anesthesiologists, Society of Cardiovascular Anesthesiologists, American College of Chest Physicians, American Association for Thoracic Surgery, American Society for Artificial Internal Organs, Society of Critical Care Medicine (SCCM), and more, with each organization linked to an exclusive journal publication. Moreover, SCCM, heralded as a hub for all intensive care, seldomly embraces CT-CCM as a unique subset of critical care [17][18][19][20][21][22][17,18,19,20,21,22]. The resulting array of independent societies and the absence of a unified entity responsible for scientific oversight in the field creates compartmentalization of knowledge, hinderance to crosspollination of ideas, and deficiency in strategic planning. Additionally, it diminishes the visibility and advocacy for the field. Consequently, the current model is not conducive to coherent, systematic inquiry necessary for promotion and expansion of CT-CCM knowledge.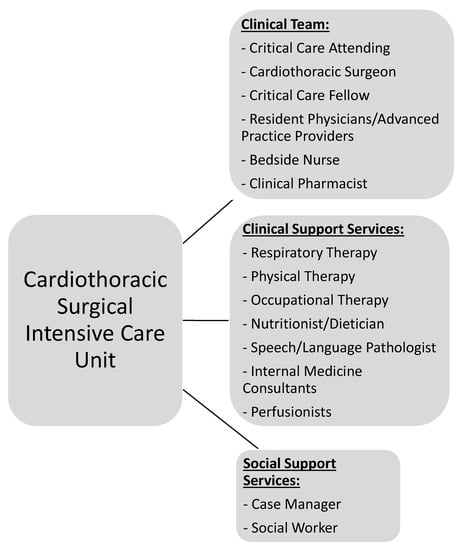
-
Cardiothoracic Surgical Critical Care Medicine is a discrete subspeciality of a medical science.
-
CT-ICU patient populations are diverse and medically unique.
-
Distinct investigations enlisting CT-ICU cohorts are required to answer basic scientific or clinical questions relating to these populations.
-
Data acquired from general medical or surgical ICU studies may not provide evidence easily translatable to CT-CCM. Application of such information should be done with caution.
-
Wide knowledge gaps exist in many areas of CT-CCM.
-
Formation of a goal setting, centralized governing body, such as CT-CCM specific society.
-
Establishment of a scientific journal centered on CT-CCM inquiry.
-
Securement of funding and development of grant programs specifically geared towards CT-CCM research.
-
Expansion of the Perioperative and Critical Care Conference co-sponsored by the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists to include other stakeholders, such as Society of Critical Care Medicine, the American Association for Thoracic Surgery, the Society of Critical Care Anesthesiologists, and the American Academy of Cardiovascular Perfusion, and more.
-
Establishment of a standardized CT-CCM training curriculum, continuing education, and certification.
3. Part 2 – Selected Gaps in Knowledge and Future Direction of Research
3.1. General Framework and Summary of Important Publications
ReseIn parcherst two of the review, we identify existing knowledge gaps affecting patients cared in cardiothoracic surgery intensive care unit (CT-ICU) and suggest a direction for further research in the field of CT-CCM. The list of topics is not exhaustive and intends to give sense of breadth and complexity of future work at hand. The discussion is divided into disorder-specific research considerations, followed by considerations for special populations. Figure 3 illustrates general framework of research needed to address CT-CCM knowledge gaps.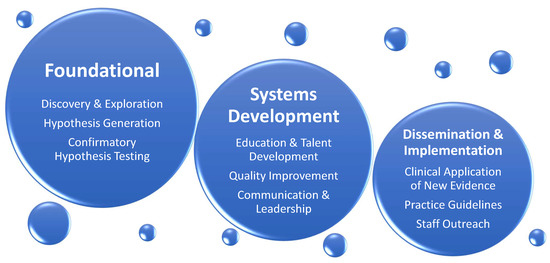
3.2. Disorder-Specific Considerations
3.2.1. Cardiac Surgery-Associated Acute Kidney Injury (CSA-AKI)
Acute kidney injury is the most common major complication occurring after cardiac surgery with incidence reaching 40% [38][76]. It remains a significant cause of morbidity and mortality even 10 years after surgery and complete renal function recovery [38][39][76,77]. Although heavily researched, more questions than answers remain, including mechanism of injury, prevention, and management [38][39][40][76,77,78]. The pathophysiology of CSA-AKI remains incompletely understood with mechanisms such as ischemia, hypoxemia, reperfusion injury, hypoperfusion, inflammation, neurohumoral activation, extracorporeal circulation, genetic predisposition, and others causally identified [39][40][77,78]. These associations have not been verified in animal models, as logistical and cost factors have prevented development of cardiopulmonary bypass (CBP) animal studies [38][76]. This calls for further scientific pressure to improve the understanding of the processes and mechanisms behind CSA-AKI; an investment into development of a CSA-AKI animal model is prudent. Many preventative and therapeutic measures have been deployed to combat CSA-AKI. Oxygen delivery, avoidance of hypoperfusion, and cardiac output augmentation continue to be the backbone of CSA-AKI prevention based the on current understanding of pathophysiology [38][41][76,79]. For example, goal-directed perfusion with oxygen delivery index >270–300 mL/min/m2 has recently been shown to improve CSA-AKI, but not in patients with high risk for postoperative AKI [41][42][43][44][45][46][79,80,81,82,83,84]. Other maneuvers have also been investigated. The Cochrane review on remote ischemic preconditioning in cardiac and major vascular surgery resulted in moderate to high certainty of no efficacy [47][85]. Additionally, most pharmacologic strategies have not proven to be helpful in preventing CSA-AKI. Therapeutics such as mannitol, steroids, dopamine, fenoldopam, sodium bicarbonate, theophylline, statins, N-acetylcystine, clonidine and antioxidant supplements have all failed to show renal-protective benefits [38][40][76,78]. Similarly, the type of resuscitative fluids used and their effects on kidney health have been debated. The majority of investigations for balanced versus normal saline crystalloid use come from mixed ICU populations, making their application to CT-ICU problematic [48][49][86,87]. Moreover, the use of albumin in continues to be controversial, with a mixed bag of results [50][88]. To date, the most significant positive results on prevention come from PrevAKI trials, where adherence to the Kidney Disease: Improving Global Outcomes (KDIGO) bundle reduced the rate of CSA-AKI in a single center RTC, and rate of moderate and severe AKI in a multicenter trial; the bundle consisted of avoidance of nephrotoxins, optimization of glycemic control, and optimization of volume status and hemodynamics [51][52][89,90]. Similarly, the ideal time of initiation of renal replacement therapy (RRT) has been in question [38][76]. Aside from well-established, life-threatening RRT indications, a single-center ELAIN trial showed benefit of early RRT initiation as compared to delayed initiation in a cohort of nearly 50% of patients recovering from cardiac surgery [38][53][76,91]. A retrospective, multicenter observational study in cardiac surgery patients showed similar results [38][76]. However, due to design flaws and lack of CT-CCM specific RTCs, the answer to early versus delayed RRT remains elusive. In summary, many questions remain unanswered regarding CSA-AKI. An intense effort is needed to elucidate the mechanisms of renal injury associated with cardiovascular surgery, effective preventative measures, and well-timed and successful therapies.3.2.2. Delirium
Delirium is the most common neurologic complication encountered in the CT-ICU occurring in 7–50% of patients [54][55][56][57][58][59][60][61][92,93,94,95,96,97,98,99]. It is also a risk factor for long-term cognitive dysfunction, functional decline, and mortality, suggesting the continued necessity for research relating to this pathological state [55][56][59][93,94,97]. Further delineation of pathophysiology and mitigation of risk factors of delirium in CT-ICU patient populations are the two areas in need of urgent inquiry important for the development of preventative and therapeutic strategies. Delirium is a syndrome with a common denominator of dysregulated neuronal function; however, systemic disturbances that produce this clinical entity are diverse [57][58][62][95,96,100]. Consequently, separate evaluation of pathophysiology of delirium in CT-CCM is required because of unique exposures such as profound hypothermia, non-pulsatile flow, or chronic hypoperfusion [58][96]. A tactic of creation of specific animal models is problematic due to difficulty of demonstrating the presence of delirium in experimental animals [57][58][95,96]. In addition, logistics and cost may be prohibitive. However, animal models with adequate face and construct validities would be helpful in identifying cellular and molecular changes [58][96]. Other innovative measures are also required to elucidate specific mechanisms behind delirium in CT-ICU populations. Further investigations into systems integration failure hypothesis, gut microbiome dysfunction, novel biomarker identification with biobank development, neuroimaging, and electrophysiology are warranted [62][63][64][100,101,102]. Additionally, a pragmatic research methodology is needed to address delirium as it relates to daily clinical practice. Tackling modifiable risk factors is most likely to yield clinically usable results. Focusing on patient-specific risks is especially important. Studies concentrating on optimizing preoperative frailty, physical conditioning, cognitive prehabilitation, nutritional status, hearing impairment, and chronic disease burden in CT-CCM populations are of utmost importance [55][57][61][93,95,99]. Cognitive prehabilitation is especially promising given encouraging results from non-cardiac surgery populations such as the Neurobics trial [65][103]. A recent feasibility trial of perioperative cognitive training in cardiac surgery established it as a viable target for further investigations [66][104]. Likewise, chronic disease burdens such as depression, arrhythmias, diabetes mellitus (DM), hypertension (HTN), stroke history, peripheral vascular disease, and obesity have been found to be statistically significant risk factors for post-CABG delirium and additional studies are needed to evaluate optimization of chronic diseases as a preventative measure [61][99]. Moreover, investigations of modifiable precipitating factors such as postoperative pain control and sedation are crucial. Both uncontrolled pain and excessive opioid administration are significantly related to delirium development [54][55][57][58][92,93,95,96]. Recent years flourished with new regional anesthetic techniques involving fascial spread of local anesthetics, allowing anesthesiologists to develop blocks covering previously inaccessible dermatomes [67][105]. Aggressive evaluations of erector spinae, serratus anterior, and transversus thoracis muscle plane blocks with randomized controlled trials (RTCs) in CT-CCM populations are needed to determine their effectiveness for optimal chest wall pain control [68][106]. Sedation choice is another modifiable risk factor needing further examination. Initial positive reports of dexmedetomidine in cardiac surgery have recently been eclipsed by strong RTCs questioning its benefit [56][60][69][70][94,98,107,108]. As a result, there continues to be no single sedative agent deemed helpful in delirium treatment or prevention in any realm of critical care. An application of volatile anesthetics for ICU sedation is on the horizon. Inhaled agents have been used for sedation with success in Europe for some time, and now, isoflurane will be evaluated in the U.S. in phase 3 clinical trials INSPiRE-ICU1&2 (NCT05327296) [71][72][109,110]. Theorized benefits include decreased opioid use, improved spontaneous breathing, shorter extubation times, and quicker wakeups [73][111]. INSPiRE-ICU 2 will also evaluate long-term cognitive outcomes. Pending results, CT-CCM cohorts will be another frontier for inhaled anesthetic inquiry. Finally, an ongoing study of lidocaine infusion for COVID-19 ARDS with secondary endpoints of delirium and opioid consumption may further aid clinicians in sedation selection and pain control (NCT04609865) [74][112]. In summary, investigational strategy into delirium in CT-CCM patient populations should follow a two-pronged response. Basic science research should address mechanisms of delirium in specific clinical scenarios, while pragmatic clinical studies should be designed to help mitigate risk factors of delirium.3.2.3. Pharmacotherapy
Gaps in knowledge exist in CT-CCM in relation to disease-specific pharmacotherapy. With the appreciation of the magnitude of the missing data, rwesearchers highlight just a few pieces of a puzzle in the following section. Patients undergoing CABG are likely the most common patient population seen in the CT-ICU. Frequently, grafts used include saphenous-vein grafts and radial artery grafts. The RADIAL trial compared saphenous vein grafts to radial artery grafts for CABG and demonstrated a significantly lower rate of adverse cardiac events in patients who received radial grafts (hazard ratio 0.67; 95% CI. 0.49–0.90; p = 0.01) [75][113]. However, the radial artery is more muscular in nature, increasing concern regarding vasospasm leading to myocardial ischemia [76][114]. As a result, the RADIAL trial had six different regimens to manage to prevent arterial graft spasm that differed in agents (diltiazem, nifedipine, or amlodipine) and duration (6 weeks to indefinite) [75][113]. Consequently, the quest for antispasmodic medications to help prevent vasospasm is ongoing. Medications that have been studied include nitroglycerin, diltiazem, verapamil, papaverine, and milrinone [77][115]. However, many of the investigations are limited to single centers with small sample sizes. Due to the lack of conclusive evidence, the ideal agent and duration of its use remain in question [78][116]. Another area with a paucity of literature is optimal antibiotic management for delayed sternal closure (DSC). Although guidelines give recommendations on agent, dose, and duration of antibiotics for prevention of surgical site infections (SSI), there are no recommendations on antibiotic therapy if primary closure is not performed [79][80][117,118]. Feared complications of DSC are the deep sternal wound infection and mediastinitis. Thus, antibiotic prophylaxis is commonly continued, but the regimens and durations vary depending on institution [81][82][119,120]. Two recent trials showed that continued administration of prophylactic antibiotics in patients with DSC were not associated with benefits in rates of mediastinitis and deep tissue infections [82][83][120,121]. Both studies were limited by their retrospective nature, single center location, and small sample size. Additional studies are needed to determine if prolonged antimicrobial prophylaxis is needed and if so, what the optimal regimen in patients managed with DSC is. An additional area that presents frequent clinical conundrums in the CT-ICU involves appropriate dosing of pharmacotherapy for patients on extracorporeal membrane oxygenation (ECMO). The available literature has revealed that patients on ECMO have an increased volume of distribution and elimination of certain medications [84][122]. Drugs with high lipophilicity or protein binding have demonstrated decreased blood concentrations, likely due to drug sequestration in the ECMO circuit, putting the patient at risk for clinical failure [85][86][123,124]. Most of the literature investigating optimal dosing are limited to ex vivo data or case reports and case series [84][122]. Due to the limitations of the literature, the provider is presented with the task of weighing the risk of an adverse drug event and the risk of clinical failure. Where this may be of most concern is the dosing of antibiotics for patients on ECMO as clinical failure could be detrimental. A recent publication comparing serum concentrations of various antibiotics in patients on ECMO versus medical therapy demonstrated a higher rate of failure to reach target concentrations of piperacillin (48.4% vs. 13.0%) and linezolid (34.8% vs. 15.0%) [87][125]. Previously, the medications that were lipophilic or highly protein-bound raised concern for therapeutic failure. However, neither piperacillin nor linezolid are particularly lipophilic with relatively low protein binding, making it less likely that sequestration in the ECMO circuit is responsible for failure to attain target concentrations. The Surviving Sepsis Campaign [88][126] recommends therapeutic drug monitoring (TDM) in critically ill patients due to alterations in volume of distribution and elimination. For patients on ECMO, TDM becomes imperative as the addition of ECMO adds another complexity to dosing considerations in an already critically ill patient. However, many institutions do not have the ability to provide TDM for many of these medications. Future studies should focus on the use of TDM for patients on ECMO and the effect of alternative dosing regimens in obtaining therapeutic goals. In conclusion, significant knowledge gaps exist in relation to pharmactotherapy specific to CT-ICU patient populations. Provided examples serve as a sample of a work ahead. However, other important pharmaceutical concepts deserve to be accounted for in this review. These include:- -
-
Therapy for vasoplegia after CBP;
- -
-
Vasopressor of choice for hypotension;
- -
-
Inotrope of choice based on pathology;
- -
-
Utility of a calcium sensitizer;
- -
-
Antibiotic therapy duration for hospital-acquired infections in cardiogenic shock;
- -
-
Pathology and mechanical support specific anticoagulation regimens and reversal agents;
- -
-
Nalaxone and spinal cord protection;
- -
-
Multimodal analgesics.
3.2.4. Transfusion and Blood Conservation
One in five cardiothoracic surgery patients will receive blood [89][127]. Unlike many fields, optimal red cell transfusion thresholds are relatively well-defined in CT surgery. For the general cardiac surgical population, a threshold of <7.5 g/dL is safe: a 5243-participant study of a <7.5 g/dL versus a <9.5 g/dL trigger for transfusion during and after moderate-to-high-risk cardiothoracic surgery found reduced transfusions and no evidence of harm in the restrictive group [90][128], with consistent outcomes at six months [91][129]. It is quite likely that a threshold of 7.0 is comparable to 7.5, but that has not been explicitly studied in large cardiac surgery trials. For non-surgical patients with active ischemia, a threshold between 7–8 g/dL is likely safe but not firmly established: a trial of 668 patients with anemia and myocardial infarction found that a transfusion trigger of <7 g/dL was noninferior to a trigger of 10 g/dL for major adverse cardiac events at 30 days—although the confidence interval for this result may include clinically significant harm [92][130]. The results of the 3500-patient Myocardial Ischemia and Transfusion (MINT) trial, expected to conclude in 2023, will likely clarify the safety of restrictive transfusions in patients with active myocardial ischemia [93][131]. Other blood products are not well-studied in the CT-ICU or any field. Triggers for plasma, cryoprecipitate, and platelet transfusion suggested by various cardiothoracic societies are reasonable but largely based on expert opinion or low-quality evidence [94][132]. Limited new data imply that the traditional emphasis on early platelet transfusion to overcome CPB-associated platelet dysfunction and consumption is misplaced and that fibrinogen repletion is more clinically effective [95][96][133,134]. Cold-stored human platelets may offer better efficacy and safety than traditional room-temperature-stored platelets, and the results from an ongoing trial in complex cardiac surgery are eagerly anticipated [97][135]. Alternatives to transfusion have also been studied: multiple meta-analyses of acute normovolemic hemodilution find that it reduces red blood cell transfusion by around 0.75 units per case and mildly reduces blood loss [98][136]. However, published trials are highly heterogeneous and the practice remains controversial [99][137]. Finally, factor concentrates may be reasonable alternatives to transfusion in cardiac surgery: a trial of 827 patients found that using fibrinogen concentrate was non-inferior to cryoprecipitate for post-CPB bleeding associated with hypofibrinogenemia [100][138], and similar studies are planned to compare 4-factor prothrombin complex concentrates with plasma [101][139]. Laboratory testing is a critical adjunct to transfusion practice in the CT-ICU. Guidelines from the Society of Cardiovascular Anesthesiologists and other organizations have placed significant emphasis of the benefits of laboratory-guided transfusion algorithms [94][132], which reduce transfusions and bleeding compared to physician judgement [102][140]. The relative benefits of specific coagulation testing platforms remain unproven and hotly debated—trials purporting to show the superiority of viscoelastic systems such as thromboelastography (TEG) over conventional coagulation tests have been confounded by the manner in which the tests were performed for the trial, e.g., comparing beside TEG to coagulation tests performed in a distant laboratory [103][141]. Well-run trials in which logistical confounders were eliminated have failed to find a relative benefit of one testing platform over another [104][142]. This suggests that testing algorithms should be designed to emphasize whatever platform is most pragmatic at the specific institution, without undue preference for a specific method. One exception to this may be in the case of a patient who has taken antiplatelet agents, where modified viscoelastic assays such as TEG Platelet Mapping may provide some useful information to guide timing and dosage of transfused platelets [105][143]. In the near future, rapid advances in point-of-care genomic and epigenetic testing may also be used to identify patients with differential responses to antiplatelet and anticoagulant medications, as well as responsiveness to transfusion therapy [106][107][144,145].3.2.5. Paralysis after Aortic Aneurysm Surgery
Patients who receive surgical or endovascular treatment for thoracic aortic aneurysms and/or dissection are often in the CT-ICU for several days to weeks. A contributing factor for longer ICU stays in these patients are post-operative complications. One of the most devastating complications of either open surgical or endovascular repair of aortic aneurysms is spinal cord ischemia and/or paralysis. Although both treatment paradigms have a risk of postoperative paralysis, there are different mechanisms which govern the pathophysiology of open aortic surgery and thoracic endovascular aortic repair (TEVAR) mediated paralysis [146]. [108]A recently published article demonstrated in a large animal model that these two mechanisms are different. Namely, the open surgical approach causes ischemic reperfusion injury versus critical permanent hypoperfusion in endovascular repair. A preventative therapeutic measure to treat these different phenomena will require an animal model that accurately maps the different pathophysiology of ischemic spinal cord injury in open and endovascular repair in humans. However, this new information needs to be confirmed in humans and other animal models. In our small and large animal models, transient aortic clamping during open surgical repair has been shown to cause primarily grey matter damage via reactive oxygen species mediated blood–spinal cord barrier disruption and leakage of intracellular contents, causing central cord edema and neuronal death. TEVAR, on the other hand, has been shown to cause white matter damage likely due to chronic hypoperfusion of segmental arteries by the stent graft. CT-ICU management of these patients currently consists of spinal cord drains to reduce spinal cord edema and the usage of vasopressors to maintain systemic perfusion pressure. The treatment of paraplegia after spinal cord ischemia requires further mechanistic clarity and basic science research to develop a pharmacologic treatment to either prevent ischemic spinal cord injury perioperatively or reverse it postoperatively. Additionally, these treatments should be tailored to the specific patient, keeping in mind the procedure they underwent and the specific mechanism of spinal cord damage. Which animal model best replicates the pathophysiology of paraplegia after open and endovascular repair? It is well known that the blood supply to the spinal cord varies across species, which raises the question of which model is the most appropriate to conduct basic science research [109][147]. Once the ideal animal model is developed to test therapeutics, there is another obstacle which researchers must consider, the mismatch which often occurs between the size of the lesion within the spinal cord observed on imaging and the functional symptoms of the lesion in the spinal cord. Simply put, lesions of similar size can result in different clinical outcomes. Termed the “neuroanatomical functional paradox”, this phenomenon is a persistent barrier to designing animal models of spinal cord injury [110][148]. This paradox makes it especially difficult to develop therapeutics, because certain regions of the spinal cord have higher levels of “eloquence” and have higher sensitivity to detect the effectiveness of treatment. On the clinical side, currently the standard of care is to drain the cerebrospinal fluid (CSF) and to manage the systemic perfusion pressure, despite low evidence and small studies demonstrating the efficacy. Even the definition of high and low-risk is not well delineated. This is a hurdle for clinicians who aim to design randomized controlled trials, given the perceived benefit of the spinal drain. Additionally, the complication associated with the spinal drain is significant, requiring clinicians to weigh the risk of prophylactic spinal drains with its benefits. A solution to this problem is a randomized controlled trial which analyzes the efficacy of prophylactic spinal drains versus their complications. Finally, there is a need for biorepositories using biological fluids (e.g., blood, CSF, urine) of patients who develop paralysis after open and endovascular aortic repair. The goal of such an endeavor will be to gain mechanistic clarity and allow researchers from many institutions to contribute to and acquire data from this repository. This can potentially fuel drug discovery targeting specific mechanisms which are involved in the pathogenesis of spinal cord injury and/or paraplegia after open and endovascular aortic repair. There is a gap in the knowledge in the field of spinal cord perfusion and that addressing the following four points is of paramount importance:- (1)
-
What is the ideal small or large animal model for open and endovascular repair of aortic aneurysms, given the variety of anatomical blood supply to the spinal cord across species?
- (2)
-
Given the neuro-radiological-anatomical functional paradox, therapeutic treatment for both disease paradigms is different and there is a need for more preclinical trials targeting the specific mechanism behind the grey- and white-matter lesions.
- (3)
-
There is a need for randomized controlled trials testing the efficacy of spinal cord drains perioperatively to prevent paralysis.
- (4)
-
There is a need for a repository containing the biological fluids of non-paralyzed patients as well as patients who develop paralysis after aortic interventions to gain mechanistic insight which will guide pharmaceutical discovery in this field.
3.2.6. Cardiac Surgical Unit—Advanced Life Support
Cardiac Surgical Unit—Advanced Life Support (CSU-ALS) was conceived as a more focused resuscitation protocol for the specific needs of post-sternotomy patients, as compared with standard ACLS. The impetus for the protocol was the recognition that external cardiac massage is best avoided or minimized in post-sternotomy patients as they are more exposed to injury from this method of artificial perfusion. In addition, the most common causes of cardiac arrest after cardiac surgery (malignant arrhythmias, bradycardia, cardiac tamponade, bleeding) are immediately reversible with appropriate recognition and treatment [111][149]. The goal of rapid re-sternotomy if initial resuscitation attempts fail is another hallmark of the protocol. The rationale for this is multifaceted. First, several common causes of arrest are either alleviated with re-sternotomy (cardiac tamponade) or more readily treatable (bleeding source recognition, epicardial pacing wire dislodgement, arrhythmias treatable by internal cardioversion). Second, the recognition of the superiority of internal cardiac massage to external massage with respect to perfusion pressures and rate of ROSC is another benefit of rapid re-sternotomy. Finally, the right ventricular injury from the posterior sternum during chest compressions has been described, calling for development of less injurious maneuvers [112][113][150,151]. The CSU-ALS protocol gained significant validation when a panel of experts from the Society for Thoracic Surgeons (STS) endorsed it in 2017 [111][149]. This prompted a wide-spread desire to implement CSU-ALS in cardiothoracic surgical ICUs world-wide. As a novel approach to resuscitation in this unique patient population, this presents significant opportunity for research and improvement on every aspect of the protocol. Given the prevalence of cardiac disease and need for surgical intervention via sternotomy, any improvements in this protocol have the potential to result in dramatic improvements in outcomes for a large patient population. In addition, with its relatively recent endorsement by the STS, there is a significant need for wider awareness and training in hospitals in the practice of the CSU-ALS protocol. Finally, strong scientific effort is necessary to further study CSU-ALS approach. Inviting American Heart Association to join future endeavors would help to achieve both, wider awareness, and research development.4. Conclusion
The demand for cardiothoracic surgical critical care will continue to expand as the burden of chronic diseases increases and the population ages. In result, utilization of CT-ICUs will escalate. This calls for reevaluation of care provided in these ICUs in preparation for the surge. Given the diversity of disciplines practicing CT-CCM, expertise specific to the field is dispersed over many different specialties, societies, and journals. Resultant compartmentalization of scientific inquiry without centralized governing body hinders growth, innovation, and patient care. For example, significant knowledge gaps exist in CT-CCM with significant portion of current CT-ICU care standards relying on data obtained from medical or general surgical patient cohorts. To improve the current model, CT-CCM needs to be recognized as a its own subspecialty and science. Additionally, subspeciality specific society, a journal publication, comprehensive annual meeting of all the stakeholders, and unified research agenda are necessary to promote its expansion. The future of cardiothoracic surgical critical care depends on realization of its distinction, unification of its professionals, realization of its knowledge gaps, and initiation of research required to answer the most pressing questions.
