During the last decades, great strides have been made in the fight against cancer. The knowledge about the mechanisms of its onset and cellular resistance has notably increased, as well as the discovery of new potential therapies. Despite this, cancer remains a problematic pathology to treat due to its characteristics of aggression, ability to metastasize, resistance onset, and severe side effects of the available drugs. For these reasons, cancer is still the second leading cause of morbidity and mortality worldwide
[1]. In the context of the most promising anticancer therapies developed so far, metal-based drugs have represented for several decades the cornerstone for tumour chemotherapy
[2][3][2,3]. In fact, platinum complexes, such as cisplatin, oxaliplatin, and carboplatin, are still the most widely used treatments in several kinds of cancer, including testicular, cervical, ovarian, and non-small cell lung cancers. However, in several cases after a variable number of chemotherapy cycles with platinum-based complexes, tumoral cells could become less susceptible to the cytotoxic effects of the drug, thanks to the activation of a multifactorial adaptive response that involves three major components: autophagy, endoplasmic reticulum stress signalling, and senescence
[4]. Thus, the majority of cisplatin-treated patients experience therapeutic failure and tumour recurrence during—or right after—the treatments
[5]. In addition, there are severe side effects associated with therapeutic regimens based on platinum complexes, such as nephrotoxicity, neurotoxicity, leukopenia, thrombocytopenia, gastrointestinal issues, nausea, vomiting, and hair loss
[6]. These latter are mainly due to poor drug selectivity causing the death of healthy cells together with the tumoral ones. Considering all these issues, improvements in developing new effective therapies can indeed be pursued. These problems have prompted researchers to focus on developing a new generation of chemotherapeutics. Firstly, the research has been extended to other transition metal compounds, such as gold, ruthenium, silver, iridium, rhenium, and copper, that proved to have important cytotoxic effects on cancer cells, becoming promising potential new anticancer drugs. For example, the gold compounds’ popularity increased following the idea to repurpose Auranofin, an Au(I)-based complex approved by the FDA in 1985 as an antirheumatic Au(I) agent, for the treatment of cancer
[7]. In fact, several studies highlighted its promising cytotoxic properties, entering also in three separate clinical trials in the United States as an anticancer compound
[8]. Additionally, for Ru-based compounds the interest of the scientific community significantly grew after the publication of NAMI-A, KP1019 and KP1339, which are three Ru(III) complexes that have entered other human clinical trials
[9]. During the past years, various strategies have been explored to optimise the efficiency of metal complexes with anti-cancer activity, both in potency and selectivity, aiming to obtain the so coveted “
anticancer magic bullet”, already conceptualised in the early 1900s by Paul Ehrlich
[10]. In this re
sview, we
arch, will focus on some examples referring to two main strategies
will be focused on: the first one regards heterobimetallic complexes with a synergic action, while the second one refers to constructs where the metallic complex was conjugated to a targeting moiety. Starting from the promising results obtained with some monometallic complexes, a new approach was attempted combining different metal centres in one compound by forming a heterobimetallic complex able to combine the various modes of action that are typical of the constituting metal centres. The heterobimetallic compound should enhance the anticancer properties of single metallodrugs. The objective is to enlighten any possible synergistic effect due to the administration of two or more active ingredients as a single drug
[11][12][11,12]. Generally, the two metal centres have a different final molecular target with diverse mechanisms of action, or they can modify different pathways in the cellular metabolism, or adjust some chemical-physical properties of the whole heterobimetallic complex, such as the solubility or lipophilicity, with different contributions
[13]. The combination of different activities into one single drug should reduce the concentration needed for the therapeutic approach, and this should decrease the side effects produced by a large amount of compound needed to have a positive response to the therapy. This behaviour could be expressed in lower IC
50 values or by overcoming the resistant problems to the current therapies. Another appealing approach involves targeting strategies, which has proved to have many advantages and potential interesting applications. Targeted anticancer agents are designed according to a strategy that aims to direct them towards cancer-specific biomarkers or biological targets which are overexpressed in cancer cells.
2. Heteronuclear Metal Complexes with Anticancer Activity
One of the new strategies here presented concerns the combination of two different metal centres in one single drug to build a heteronuclear metal complex. The combination of more than one metal was already used for other types of applications, such as catalysis, where electronically different metals could synergistically cooperate and have better properties compared to the monometallic precursors in a catalytic process
[14][15]. The same approach was used more recently for potential anticancer applications. The opportunity to use different metal-based compounds, which act as anticancer but have different mechanisms of action from each other, could promote cooperative activity and synergistic effects. In this re
svie
arch, researchersw, we divided different examples into two main groups, one of platinum and one of gold. These two metal centres, as monometallic compounds, have been extensively studied and they are known to exploit their anticancer activity in different types of final targets. Platinum compounds generally act in the genomic moiety
[15][16], while gold complexes are considered good inhibitors of thioredoxin reductase
[16][17]. One of the most recent and interesting results of the literature concerns the combination of platinum or gold with different metal centres or a combination of them. A discussion on the possible synergistic effects of the new heteronuclear metal drugs has been done. To be sure that the enhanced anticancer activity is truly done by the cooperative action of the two metal centres, a comparison with the monometallic fragments is always needed.
2.1. The Case of Platinum
Transition metal complexes have been deeply studied for different applications, such as catalysis, antimicrobial, and also antitumoral drugs
[17][18]. Platinum complexes are probably the most well-known and studied in the field of anticancer compounds, also thanks to the omnipresence of cisplatin and its derivatives as a starting point
[18][19][19,20]. To overcome all the side effects of cisplatin such as neurotoxicity, nephro-, oto-, and gastrointestinal toxicity, alternative transition metal centres gained a place in this field
[20][21][22][21,22,23]. In the last years, there are many examples of Pt(II) complexes combined with different metal centres such as Ru(II), Rh(III), Au(I), Re(I), Tc(I)
[16][23][24][25][26][17,24,25,26,27]. Platinum-based complexes are mainly binders of genomic targets via a covalent bond between the Pt metal centre and N-7 atom of guanine base
[15][16], even if there is also evidence of protein binding, as serum albumin
[27][28]. Here
reswe
archers present the most recent and promising results of bimetallic complexes that involve different types of compounds such as photoactivable, macrocycle, or prodrugs compounds. The possible synergistic effects of two metal centres with different targets were evaluated.
Starting from the ruthenium and rhodium ones, these metal centres can form organometallic complexes usually by the coordination to nitrogen atoms or with arene ligands, making the coordination easily achievable
[28][29]. Ruthenium-based compounds, usually considered to have antimetastatic activity, received interest as anticancer agents after the discovery of the antitumoral properties of NAMI-A, KP1019, and KP1339 mentioned above. These organometallic Ru(II)-arene derivatives have a different mechanism of action compared to cisplatin to exploit the anticancer activity, which involves the binding to the biomolecule transferrin, an iron-binding protein
[29][30].
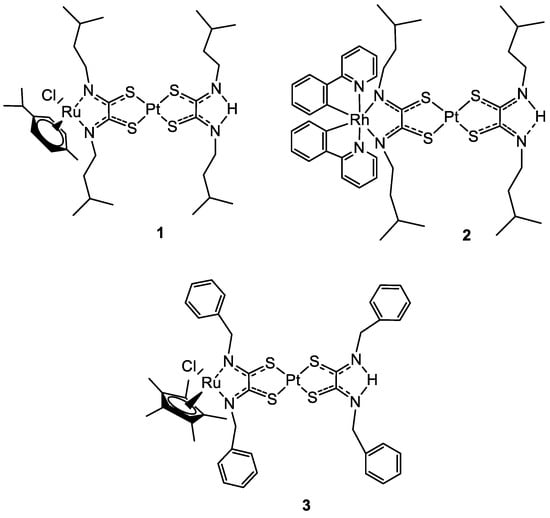
In 2019 Askari and co-workers published their work, where the synthesis of three heterobimetallic Pt(II)-Ru(II) and Pt(II)-Rh(III) complexes (
1–
3) (
Figure 1) is described. The three bimetallic complexes
1–
3 turned out to be active in the human 20S proteasome, one of the main targets for cancer therapy. Compounds
1–
3 also inhibited cathepsin B and L, which take part in tumour progression and invasion. The cytotoxic activity has been evaluated also in comparison to the Pt(II)-based precursors and cisplatin. The bimetallic complexes showed a good reduction of cell viability in different tumour cell lines such as neuroblastoma SH-SY5Y, melanoma SKMel-28, hepatocellular adenocarcinoma HepG2, and colorectal adenocarcinoma Caco-2 tumour cell lines, comparable or in some cases even greater than cisplatin, while for the healthy cell line of human fibroblasts WI 38 there is no sign of cytotoxicity. The flow cytometry analyses with Annexin-V/PI staining revealed that the cell death mechanism in the SH-SY5Y line is apoptotic after 72 h of treatment. Pt(II) precursors showed no significant inhibition or antiproliferative activity, which makes them a structural feature of the proposed heterobimetallic complexes
[24][25].
Figure 1.
Molecular structure of compounds
1
–
3
.
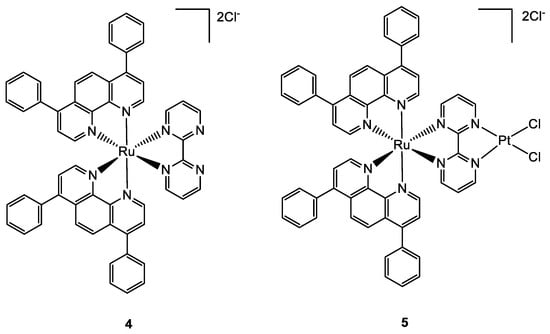
A paper by Zheng and co-workers, published in 2018, described the synthesis and characterization of a new bimetallic Ru(II)-Pt(II) complex (
Figure 2). In this case, the platinum moiety plays a pivotal role in the activity of the whole bimetallic compound improving the anticancer activity of the Ru-containing moiety. The photoactivable Ru complex
4 is combined with cis-Pt(DMSO)
2Cl
2 to obtain the heterobimetallic complex
5 through the coordination of Pt to the two free nitrogen of the bpm (2,2′-bipyrimidine) ligand. The dichloroPt(II)-containing portion could covalently bind DNA. All the tests have been performed both in dark and light conditions. The cytotoxic activity of
5 is extremely increased in light conditions and is more cytotoxic compared to
4. These results pointed out that both complexes have overcome the cisplatin resistance of the A549R (lung cancer) cell line taking advantage of the photodamage of mtDNA. Moreover, the bimetallic complex
5 can bind to mtDNA also through the Pt centre; in fact, the impairment of the polynucleotide occurs both in the dark and upon visible light irradiation. The higher affinity of
5 to DNA is due to the double type of binding: covalent, through the Pt(II) moiety, and noncovalent, through the planar ligand of Ru(II) moiety. Under light irradiation,
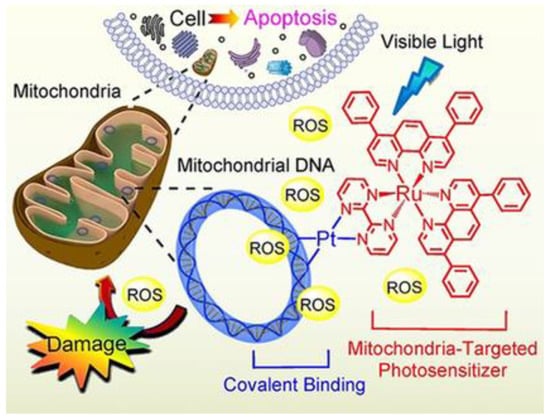
5 can produce ROS inside the mitochondria, causing their dysfunction, including MMP collapse, ATP depletion, and attenuation of mitochondrial energy status (see
Figure 3). Both complexes have been tested in vivo and compound
5 has shown better pharmacological properties with respect to compound
4. In particular,
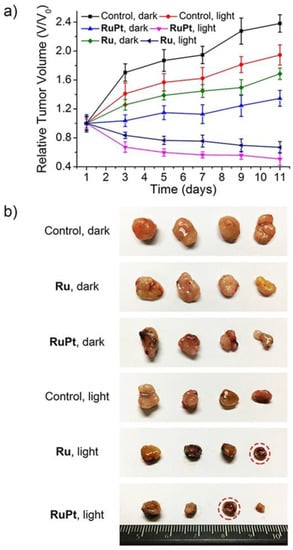
5 produced lower side effects and decreased the tumour volume without causing mouse death (
Figure 4)
[30][31].
Figure 2.
Molecular structure of compounds
4
and
5
.
Figure 3. Representation of the mechanism of action of complex 5. (Reprinted with permission from ref.
[30][31]. Copyright 2018 John Wiley and Sons).
Figure 4. (
a) Chart of the relative tumour volumes (V/V
0) of mice treated with PBS,
4 (Ru), and
5 (RuPt), measured for 11 days after treatment with PBS (control), under dark and light conditions. On the first and sixth days, intratumoral injections were performed. (
b) Photo of the tumours removed from the nude mice. The completed scavenged tumours have been underlined by red dotted circles. (Reprinted with permission from ref.
[30][31]. Copyright 2018 John Wiley and Sons).
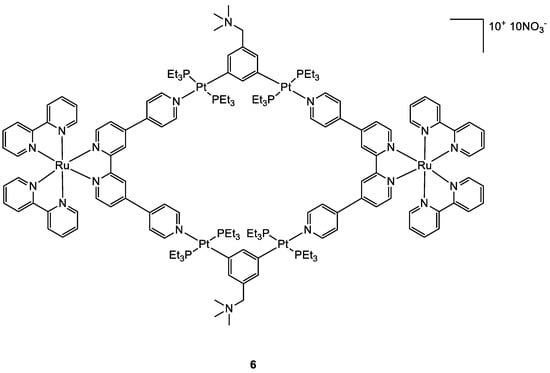
Photoactivable systems seem to be a good alternative to overcome cisplatin toxicity and another promising new metal complex that is active upon irradiation inside the tumour cells has been studied by Zhou and co-workers
[31][32]. The Ru–Pt complex
6 (
Figure 5) has a large macrocyclic structure, and the metallacycle facilitates its cellular uptake, as confirmed by ICP-MS analyses. The uptake values for complex
6 reach 114.82 ng and 451.37 ng per million cells for Ru and Pt, while the same test with the Ru moiety alone revealed a maximum cellular Ru concentration of 2.32 ng per million cells. The study of the cytotoxic activity on the A549 cell line showed that
6 is accumulated preferentially in mitochondria and nuclei as an intact metallacycle (confirmed by confocal laser scanning microscopy analysis). Compound
6 exhibits its cytotoxic potential upon LED irradiation at 450 nm (21.8 mW cm
−2, 5 min), with IC
50 values of 0.71–4.4 μM, while it is extremely decreased in dark conditions, with IC
50 of 65.2–80.9 μM. Light promotes the generation of singlet oxygen specie which causes cell death producing damages concurrently in the mitochondria and nuclei. As already observed for products
5 and
4, also in this case the in vivo tests at low light doses obtained with a diode laser (800 nm, 50 mW, 20 s/mm), showed that
6 causes an effective tumour remission associated with low systemic toxicity.
Figure 5.
Molecular structure of compound
6
.
Another interesting strategy, that involves in this case the platinum moiety, is to exploit the advantage related to Pt(IV) complexes. The platinum centre in its higher oxidation state is chemically less reactive and kinetically more inert than its respective Pt(II) counterpart. This feature can be fruitfully used to prevent off-target reactions, obtaining complexes that can be considered prodrugs
[32][33][33,34]. Moreover, the Pt(IV) complexes adopt the octahedral coordinative geometry, giving the opportunity to functionalise the axial positions with other biologically active molecules
[34][35][35,36]. Since inside the cancer cells there is an increased reductive environment compared to the healthy ones
[36][37], this can be exploited to obtain the selective Pt(IV) reduction inside the cancer cell, restoring the reactive Pt(II) complex with the simultaneous release of the axial ligands
[37][38].
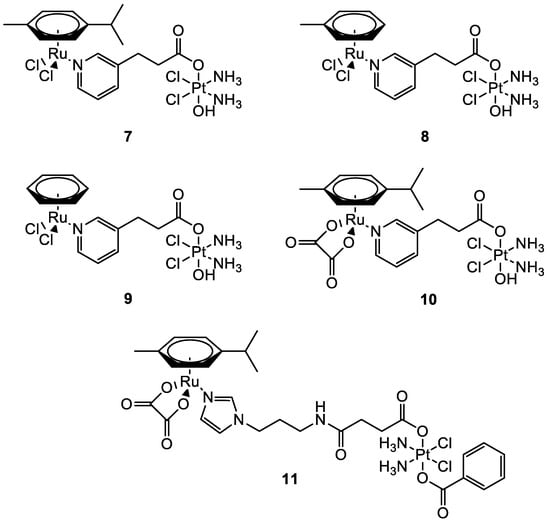
In this frame, the Guangyu Zhu group worked on the synthesis of new Pt(IV)–Ru(II) metal complexes (
Figure 6) which combine the cytotoxic properties of cisplatin with the antimetastatic properties of ruthenium-arene complexes. They were all found to be more cytotoxic compared to cisplatin with low micromolar (
11), sub-micromolar, and nanomolar (
7–
10) cytotoxicity in most of the human cancer cells tested. All the bimetallic complexes are effective also in cisplatin-resistant cells A2780cisR (ovarian carcinoma) and A549cisR (lung adenocarcinoma). Compounds
7–
11 have a selectivity index (the ratio between the IC
50 in MRC-5 (lung fibroblast) and the IC
50 in A549 cells) that is nearly 10-fold higher than that of cisplatin. A direct comparison with the performances of the monometallic moieties revealed that the increased cytotoxic activity is produced by a synergistic effect between the two metal complexes
[38][39][39,40].
Figure 6.
Molecular structure of compounds
7
–
11
.
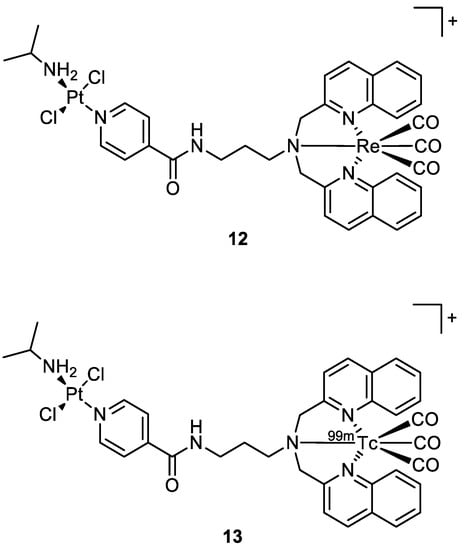
Quental and co-workers investigated the combination of two different metal centres: one with therapeutic properties (i.e., Pt(II)), and the other with properties useful in diagnostics (i.e., Re(I)) to achieve a theranostic agent. The new heterobimetallic complex (
12) (
Figure 7) had indeed a rhenium moiety with photosensitizing properties and a non-classical platinum centre with intrinsic cytotoxic activity in the dark. The metalation of cellular DNA caused after the treatment with
12 both in the dark or under irradiation was investigated by employing ICP-MS analysis. After the administration of
12 in dark conditions, the polynucleotide was extracted from the cell culture and mineralised, and ICP-MS detected no presence of platinum. This agrees with gel electrophoresis experiments, where there is no modification of the mobility of the supercoiled form. On the contrary, light exposure of the cell culture treated with the studied compound increases the amount of open circular form, which is correlated to photosensitization with consequent ROS production (singlet oxygen
1O
2).
Figure 7.
Molecular structure of compounds
12
and
13
.
The Re-tricarbonyl unit in
12 could be replaced by
99mTc (
13) (
Figure 7) to allow biodistribution investigations and imaging studies in normal mice. The intensity of radioactivity was higher in the excretory organs (liver and kidney), where
13 accumulated the most
[26][27].
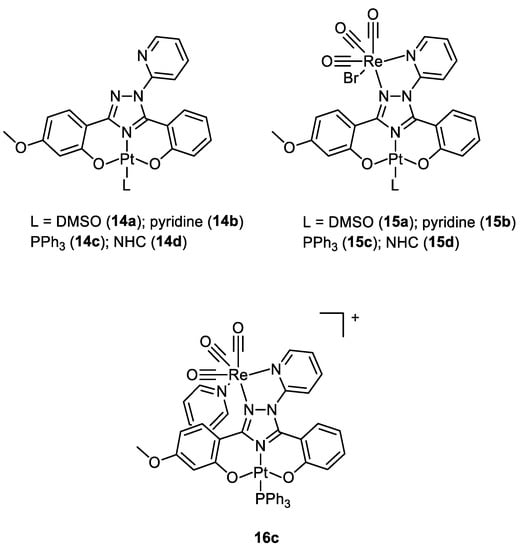
In 2020, Bertrand and co-workers synthesised different bimetallic complexes of Pt(II)-Re(I) (
15a–
d,
16c) starting from the Pt(II) precursors (
14a–
d) (
Figure 8). The first screening was based on the MTT assay (
Table 1). The most promising ones were the monometallic
14a and the bimetallic
15a,
15d, and
16c which were 10 times more cytotoxic compared to cisplatin. Additionally,
15b and
15c had no cytotoxic activity, while
16c was the most cytotoxic of this group. Even those that showed good IC
50 values on MDA-MB-231, MCF-7, and A2780 cancer cell lines all showed a very scarce selectivity when comparing the data obtained with the control healthy cells (MCF-10A). In addition, the lipophilicity of the whole complex (
Table 1) was directly correlated to the ligand nature, and the bimetallic compounds
15a–
d were more lipophilic than the mono Pt counterparts, while the charged
16c was the most hydrophilic. The amount of metal content revealed in cellular uptake experiments exactly reflected this order of lipophilicity, and it was not correlated to the cytotoxic activity. Additionally, in this case, there was no evidence of synergistic effects
[23][24].
Figure 8.
Molecular structure of compounds
14a
–
d
,
15a
–
d
, and
16c
.
Table 1. Antiproliferative activity of compounds
14a,
15a,
15d,
16c, and cisplatin on different cancer cell lines and the healthy cell line MCF-10F after 72 h of incubation at 37 °C. Partition coefficients of all complexes are reported as Log P
o/w. (Adapted with permission from ref.
[23][24]. Copyright 2020 John Wiley and Sons).
2.2. The Case of Gold
Other types of combinations involve gold compounds. Gold complexes are usually composed by the coordination of the soft metal centre with phosphine or sulphur ligands, but carbenes ligands have also gained much interest
[41][42][42,43]. Gold complexes express anticancer activity by disrupting the reduction/oxidation (redox) system within the cell via the inhibition of thioredoxin reductases (TrxRs). This enzyme is present in the mitochondria and an alteration of its activity could lead to apoptosis
[16][43][44][45][46][17,44,45,46,47]. Here
rwe
searchers present some relevant examples of recently synthesised complexes that combine the cytotoxic potential of gold-based compounds with other metal centres which exploit their anticancer activity differently, such as Pt(II), Ru(II), Ti(IV), Ir(III), and Re(I).
To date, there are only a few examples in the literature on the combination of Au(I) and Pt(II) metal centres
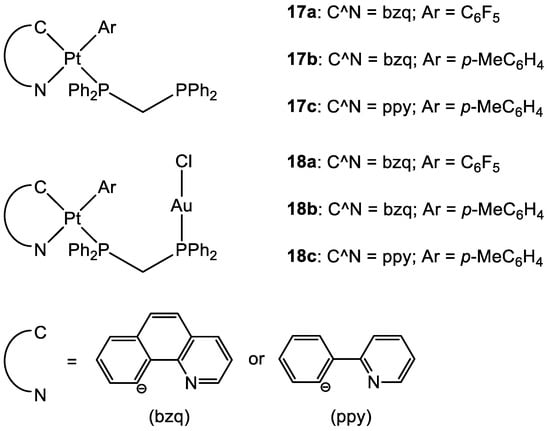
[13][47][48][13,48,49]. Some of the most promising examples are the heterometallic Pt–Au complexes depicted in
Figure 9 (
18a–
c), which have shown synergistic effects by an improvement of the antiproliferative activity against A549 (lung), SKOV3 (ovarian), and MCF-7 (breast) cancer cell lines in comparison to their Pt-based precursor complexes
17a–
c and [ClAu(μ-dppm)AuCl], which are not toxic to any of the cell lines investigated. Inhibition of cell proliferation was also compared to cisplatin and Auranofin, revealing
18b as the most promising complex, and apoptosis is the cell death mechanism in MCF-7 cancer cells in a dose-dependent manner. Furthermore,
18a effectively internalises in MCF-7 cells, and the nucleus showed the highest accumulation of the complex, which was revealed by fluorescence microscopy, with less dispersion in the cytoplasm. Selectivity for cancer cells was investigated by comparing the IC
50 values of the healthy cell line MCF-10A (epithelial breast), with the one obtained with MCF-7. All the complexes showed good selectivity for the tumorigenic cell line, and complexes
18b and
18c demonstrated higher specificity for human breast cancer cells with minor damage to normal epithelial breast cells
[13].
Figure 9.
Molecular structure of compounds
17a
–
c
and
18a
–
c
.
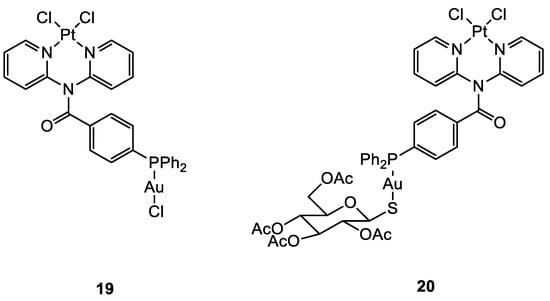
In the case of the two Pt(II)-Au(I) complexes (
19, 20) (
Figure 10) proposed by Wenzel and co-workers, the new compounds exhibited great cytotoxic activity. A comparison of the IC
50 values between the ovarian cancer cell A2780 and cisplatin-resistant A2780cis showed that
19 and
20 were able to overcome possible resistance phenomena. There is no evidence of cooperative effects of the two metals, and the cytotoxicity towards a healthy cell line is comparable to the tumoral one, demonstrating a total lack of selectivity. The sugar moiety seems to improve the transport of the compound into the cells, but the phosphine ligand may counter the Pt moiety from freely binding to the double strand of DNA
[48][49].
Figure 10.
Molecular structure of compounds
19
and
20
.
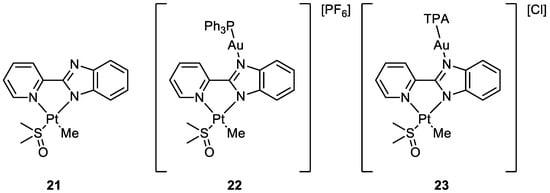
Cinellu and co-workers compared the activity of monometallic Pt(II) complex
21 with the bimetallic Pt(II)-Au(I) complexes
22 and
23 (
Figure 11). Interestingly, the heterobimetallic complex bearing the triphenylphosphine ligand (
22) presented greater cytotoxic effects compared to the mononuclear Pt complex
21. The IC
50 value obtained after the administration of
21 and the gold(I) precursor (AF) together in an equal amount was compared with the values obtained with the heterobimetallic complexes. The data suggested an additive rather than a synergistic effect of the platinum and gold centres in compound
21. On the other hand, it is important that even if joined in the same molecular scaffold, the two metal complexes do not reduce their biological activity. Further, the Au(TPA) ligand decreases the antiproliferative activity of complex
23. Moreover, compounds
21 and
22 interact with RNase A but in a different way. It seems that Rnase A shows selectivity for gold over platinum. In addition, complex
22 breakdowns in the presence of the oligonucleotide (ODN4: 5′-CGC-GCG-3′), forming different adducts with the pt fragment, [AuPPh
3]
+ and Au(I). The formation of different biomolecular adducts, which could be caused by the presence of different metal centres in the same molecule, may lead to enhanced cytotoxic effects in agreement with the observed additive effect of coupled metals
[47][48].
Figure 11.
Molecular structure of compounds
21
–
23
.
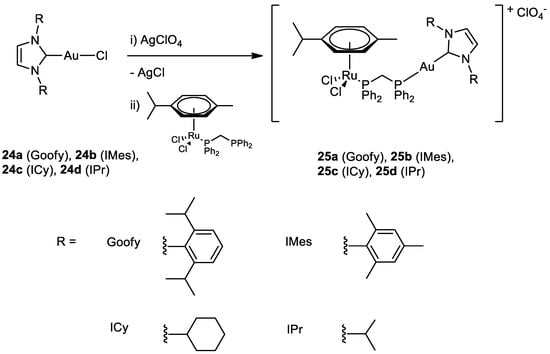
The group of Maria Contel synthesised new heterobimetallic Ru(II)-Au(I) complexes (
25a–
d) (
Figure 12) where the Au metal centre is coordinated to an N-heterocycle carbene. PrestoBlue assay, a resazurin-based compound which has similar behaviour to the most well-known MTT, was used to evaluate the antiproliferative activity of the complexes
[49][50]. The bimetallic complexes are more cytotoxic compared to cisplatin and have a better selectivity toward cancer cell lines compared to the healthy ones (HEK293T). The same assay was performed with a combination of a ratio of 1:1 of the monometallic gold (
24a–
d) and ruthenium counterparts and they all have a lower IC
50 value compared to the corresponding heterometallic complexes. Compounds
25a–
d do not interact with the DNA plasmid pBR322, so other targets may cause cell death. Complex
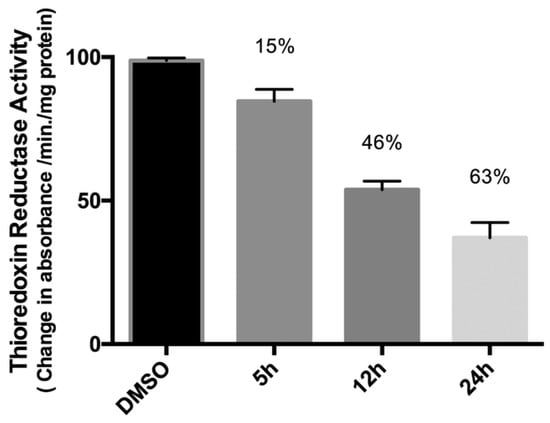
25a has been tested for the inhibition of the TrxR (thioredoxin reductase) and it is more efficient than its monometallic counterparts (
Figure 13). This behaviour could suggest a synergist effect of the two metal centres when present in the same compound
[50][51]. Further inhibition studies on complex
25b published two years after revealed as inhibited molecular targets different interleukins, metalloproteases, and cathepsins, which are important in tumour metastasis and angiogenesis
[51][52].
Figure 12.
Molecular structure of compounds
24a
–
d
and
25a
–
d
.
Figure 13. Activity of thioredoxin reductase of Caki-1 cells treated with 0.1% of DMSO (control) or complex
25a (5 µM) after 5, 12, and 24 h of incubation. (Reprinted with permission from ref.
[50][51]. Copyright 2016 Royal Society of Chemistry).
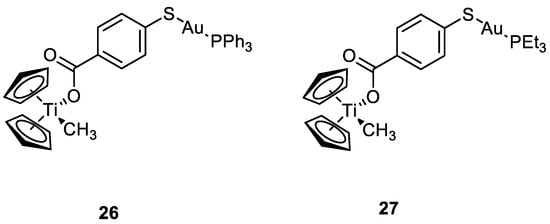
The same group worked also with heterobimetallic complexes of Ti(IV)-Au(I) (
26 and
27) (
Figure 14). The mechanism of action of
26 and
27 is different compared to cisplatin, because they do not bind DNA, but they inhibit protein kinases such as p90-RSK, AKT, MAPKAPA, and thioredoxin reductase
[52][53][53,54]. The two new compounds show IC
50 values under the µM range, after 72 h of incubation, for renal cancer cell line Caki-1. Compounds
26 and
27 are more cytotoxic than the Au(I) monometallic derivatives and much more cytotoxic than the titanocene dichloride, with effective anti-migration and anti-invasive properties, making these bimetallic compounds two promising candidates for cancer therapy
[54][55].
Figure 14.
Molecular structure of compounds
26
and
27
.
Ir(III) complexes can form emissive phosphorescent compounds with tuneable photophysical properties. By changing the organic ligands, the wavelength of emission changes accordingly, obtaining highly pure primary colours for OLEDs (full-colour displays), or near-infrared emission for chemical sensors, bio-imaging, and telecommunication
[55][56][56,57]. Only very recently have Ir(III) complexes exhibited their potency as theranostic agents. Their emission properties enable the visualisation of their cellular biodistribution and, concomitantly, Ir(III) complexes have displayed good cytotoxicity towards different cancer cells
[57][58][58,59].
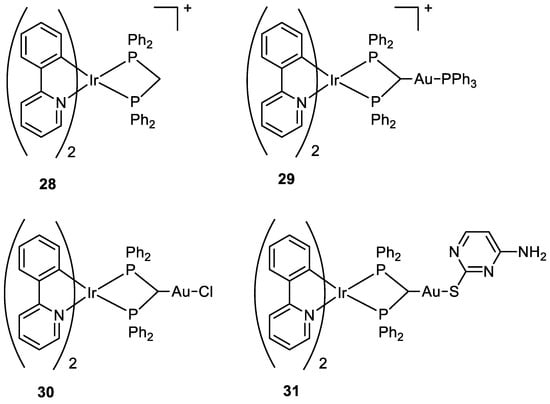
Three new Au(I)-Ir(III) bimetallic complexes (
29, 30, and
31) (
Figure 15) were presented by Redrado and co-workers in 2021 and the results were compared with the monometallic precursor [Ir(N^C)
2(dppm)]
+ (
28). All complexes induce apoptosis with the formation of cytoplasmic vacuolisation. The value of IC
50 in A549 cells ranges between 0.6 and 1.7 µM, with complex
31 being the most cytotoxic. The emission properties of Ir(III) moiety (green irradiation) allowed the investigation of cell distribution, which showed the complexes more located in the cytoplasm, and the superimposition with MitoTracker Red (MTR) emission (red irradiation), a mitochondrial selective dye used as internal standard, suggested an accumulation in the mitochondria. The presence of the gold fragment increases the generation of ROS and the inhibition of TrxR but there is no evidence of synergistic or additive effects for the heterometallic complexes
[59][60].
Figure 15.
Molecular structure of compounds
28
–
31
.
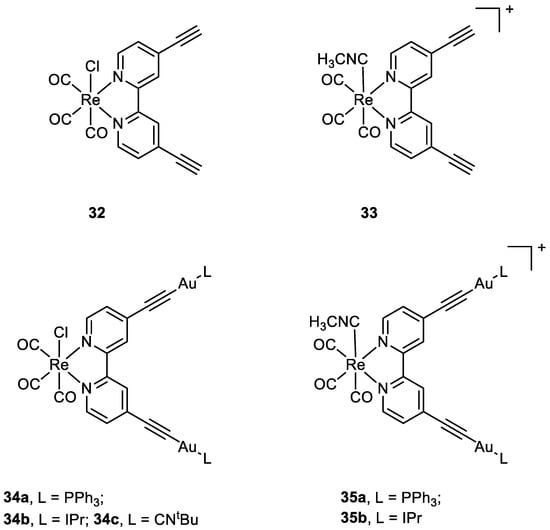
Luengo and co-workers published a paper on Au(I)-Re(I) heterometallic complexes (
34a–
c and
35a–
b) (
Figure 16). The antiproliferative activity has been evaluated with lung cancer cells A549 and cervix cancer cells He-La. Better results have been obtained for the cationic complexes compared to the neutral ones. The monometallic Re(I) complexes (
32, 33) show their cytotoxic activity even at 24 h, while only the heterotrimetallic complexes reveal their selectivity towards HeLa cells after 72 h with exception of
34b. The gold moiety is involved in the mechanism of action, even in terms of internalisation. The gold ancillary ligand guides the cellular uptake following the order -PPh
3>-CN
tBu>-Ipr. The distribution of the heterometallic species in HeLa cells was evaluated with fluorescent microscopy, thanks to the optical properties of Re moiety. In A549 cells all the compounds precipitate and accumulate outside the cells, especially the neutral complexes
32 and
34a–
c, while in HeLa cells, the positively charged heterotrimetallic compounds
35a–
b were in the outer cellular membrane media, very close to the membrane, suggesting a possible interaction of the complexes with the membrane. These different behaviours may explain the selectivity and higher toxicity toward the HeLa cell line over A549. The monometallic Re complex
33 internalised in the cells and no precipitate was detected outside the cell membrane. This suggests that the gold moiety could be responsible for the localisation pattern, additionally with the selectivity for HeLa cells membrane over A549 one, displayed by heterometallic species
[60][61].
Figure 16.
Molecular structure of compounds
32
,
33
,
34a
–
c
, and
35a
–
b
.