Chemical communication is very important in herbivorous insects, with many species being important agricultural pests. They often use olfactory cues to find their host plants at a distance and evaluate their suitability upon contact with non-volatile cues. Responses to such cues are modulated through interactions between various stimuli of biotic and abiotic origin. In addition, the response to the same stimulus can vary as a function of, for example, previous experience, age, mating state, sex, and morph.
- insect herbivore
- behavior
- pest control
- host recognition
- long- and short-range recognition
1. Chemical Cue Interaction Leads to Plasticity in Host Plant Recognition
2. Chemical Host Plant Recognition Is Modulated by Visual Cues
3. Experience-Dependent Plasticity in Host Plant Responses
3.1. Plasticity Due to Olfactory Experience
3.2. Learning of Odors through Association with Gustatory Signals
3.3. Plasticity in Contact Compound Responses through Gustatory Experience
4. Influence of Physiological State on Host Plant Responses
4.1. Nutritional State and Symbiotic Bacteria can Modify Responses to Food- and Host-Related Volatiles
4.2. Pronounced Effects of Age and Mating State on Female Responses to Host-Related Volatiles
4.3. Sex-Dependent Responses to Host Plant Volatiles
4.4. Morph-Dependent Responses to Host Plant-Related Volatiles
5. Implications of chemosensory plasticity for alternative pest management
Efforts to replace classical pest management strategies using synthetic insecticides with alternative strategies have been made since many years. However, recent developments in the banning of hazardous insecticides require more research on alternative pest management strategies, and chemical ecology provides promising avenues for behavior-based control strategies using plant compounds. Such methods comprise the use of repulsive and attractive compounds in dispensers and/or companion plants and possibly their essential oils, either in combination with confined insecticides in an attract and kill approach, or the combination of repulsive and attractive elements in push–pull systems. Other approaches include masking of host plant compounds to disrupt recognition, selection of crop varieties emitting less attractive volatiles, and manipulation of volatile emission in plants by plant resistance induction through plant defense stimulators [108,109][53][54].
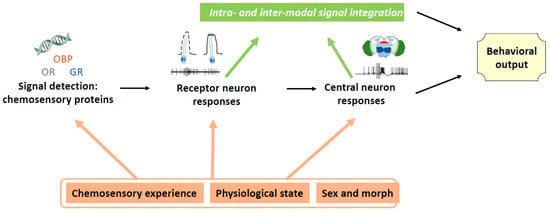
Chemical signals, alone or in concert with other sensory cues, are essential for host plant recognition and choice, but determining the identity of attractive or repulsive compounds is far from being sufficient to develop efficient and reliable crop protection methods based on these signals. First, collection of potentially active volatiles needs to be performed at the correct physiological state of the host or non-host plant. Second, the complexity of the signal and interactions between various host plant cues need to be considered. Indeed, as shown in the present review, there are multiple interaction levels between various host plant cues and their environment (Figure 1). The knowledge on chemical cue interactions is important for potential applications, because the use of attractive or repulsive plants is costly both in time and money, and therefore, as a first step, identification of individual volatile compounds, which can potentially be produced at a reasonable cost and applied relatively easily is often attempted. Nevertheless, it is difficult to reach high efficiency with this approach.

Figure 1.
Overview over the different origins of plasticity in host plant recognition.
Furthermore, as shown in the present review, insect behavior is highly plastic (Figure 1) and therefore the different forms of plasticity need to be taken into account when developing pest control methods involving the chemical senses. Insects with the same genome might not respond in the same way to chemical stimuli as a function of various factors, such as experience, physiological state, and season of the year. Powerful attractants or repellents might change their efficiency through adaptation and learning processes, as shown for the above-described case of a switch in the effect of sucrose from a phagostimulant to an aversive substance in cockroaches experienced with toxic baits [64]. Attractive, repellent, or stimulating compounds might not have the same effects on mature and immature insects.
This Hereview, it shows that mechanisms of insect host plant localization and recognition are highly plastic. These mechanisms are an excellent target for the development of alternative pest control methods. Some of these are already widely used but have a significant potential to be further improved and extended to a large range of pests. In particular, their use may have been oversimplified, while we show hereit is showed that plasticity both among plant cues or in behavioral responses of insects may strongly influence their efficiency and durability of use. Based on the rich literature reviewed in the present paper, we herein, there is a hope to see consideration of plasticity in future research on next-generation, sustainable, pest insect control based on behavioral manipulation.
References
- Conchou, L.; Lucas, P.; Meslin, C.; Proffit, M.; Staudt, M.; Renou, M. Insect Odorscapes: From Plant Volatiles to Natural Olfactory Scenes. Front. Physiol. 2019, 10, 972.
- Schröder, R.; Hilker, M. The Relevance of Background Odor in Resource Location by Insects: A Behavioral Approach. BioScience 2008, 58, 308–316.
- Randlkofer, B.; Obermaier, E.; Hilker, M.; Meiners, T. Vegetation Complexity—The Influence of Plant Species Diversity and Plant Structures on Plant Chemical Complexity and Arthropods. Basic Appl. Ecol. 2010, 11, 383–395.
- Thiery, D.; Visser, J.H. Masking of Host Plant Odour in the Olfactory Orientation of the Colorado Potato Beetle. Entomol. Exp. Appl. 1986, 41, 165–172.
- Togni, P.H.B.; Laumann, R.A.; Medeiros, M.A.; Sujii, E.R. Odour Masking of Tomato Volatiles by Coriander Volatiles in Host Plant Selection of Bemisia tabaci Biotype B. Entomol. Exp. Appl. 2010, 136, 164–173.
- Raffa, K.F.; Andersson, M.N.; Schlyter, F. Chapter One Host Selection by Bark Beetles Playing the Odds in a High-Stakes Game. Adv. Insect Physiol. 2016, 50, 1–74.
- Barman, J.C.; Campbell, S.A.; Zeng, X. Exposure to Guava Affects Citrus Olfactory Cues and Attractiveness to Diaphorina citri (Hemiptera: Psyllidae). Environ. Entomol. 2016, 45, 694–699.
- Ninkovic, V.; Glinwood, R.; Dahlin, I. Weed-Barley Interactions Affect Plant Acceptance by Aphids in Laboratory and Field Experiments. Entomol. Exp. Appl. 2009, 133, 38–45.
- Dardouri, T.; Gautier, H.; Costagliola, G.; Gomez, L. How French Marigold (Tagetes patula L.) Volatiles Can Affect the Performance of Green Peach Aphid. Integr. Prot. Fruit Crops IOBC-WPRS Bull. 2017, 123, 71–78.
- Campbell, S.A.; Borden, J.H. Additive and Synergistic Integration of Multimodal Cues of Both Hosts and Non-Hosts during Host Selection by Woodboring Insects. Oikos 2009, 118, 553–563.
- Piñero, J.C.; Jácome, I.; Vargas, R.; Prokopy, R.J. Response of Female Melon Fly, Bactrocera cucurbitae, to Host-Associated Visual and Olfactory Stimuli. Entomol. Exp. Appl. 2006, 121, 261–269.
- Bolton, L.; Pinero, J.; Barrett, B.A. Olfactory Cues From Host- and Non-Host Plant Odor Influence the Behavioral Responses of Adult Drosophila suzukii (Diptera: Drosophilidae) to Visual Cues. Environ. Entomol. 2021, 50, 571–579.
- Volpe, H.X.L.; Zanardi, O.Z.; Magnani, R.F.; Luvizotto, R.A.G.; Esperança, V.; de Freitas, R.; Delfino, J.Y.; Mulinari, T.A.; de Carvalho, R.I.; Wulff, N.A.; et al. Behavioral Responses of Diaphorina citri to Host Plant Volatiles in Multiple-Choice Olfactometers Are Affected in Interpretable Ways by Effects of Background Colors and Airflows. PLoS ONE 2020, 15, e0235630.
- Wenninger, E.J.; Stelinski, L.L.; Hall, D.G. Roles of Olfactory Cues, Visual Cues, and Mating Status in Orientation of Diaphorina citri Kuwayama (Hemiptera: Psyllidae) to Four Different Host Plants. Environ. Entomol. 2009, 38, 225–234.
- Anderson, P.; Anton, S. Experience-Based Modulation of Behavioural Responses to Plant Volatiles and Other Sensory Cues in Insect Herbivores. Plant Cell Environ. 2014, 37, 1826–1835.
- Anderson, P.; Sadek, M.M.; Larsson, M.; Hansson, B.S.; Thöming, G. Larval Host Plant Experience Modulates Both Mate Finding and Oviposition Choice in a Moth. Anim. Behav. 2013, 85, 1169–1175.
- Proffit, M.; van Dam, N.; Khallaf, M.A.; Carrasco, D.; Larsson, M.C.; Anderson, P. ‘Do You Remember the First Time?’ Host Plant Preference in a Moth Is Modulated by Experiences during Larval Feeding and Adult Mating. Ecol. Lett. 2015, 18, 365–374.
- Thöming, G.; Larsson, M.C.; Hansson, B.S.; Anderson, P. Comparison of Plant Preference Hierarchies of Male and Female Moths and the Impact of Larval Rearing Hosts. Ecology 2013, 94, 1744–1752.
- Lhomme, P.; Khallaf, M.; Larsson, M.; Anderson, P. A Sensitive Period for the Induction of Host Plant Preference in a Generalist Herbivorous Insect. Anim. Behav. 2020, 169, 1–8.
- Rösvik, A.; Lhomme, P.; Khallaf, M.A.; Anderson, P. Plant-Induced Transgenerational Plasticity Affecting Performance but Not Preference in a Polyphagous Moth. Front. Ecol. Evol. 2020, 8, 254.
- Moreau, J.; Rahme, J.; Benrey, B.; Thiery, D. Larval Host Plant Origin Modifies the Adult Oviposition Preference of the Female European Grapevine Moth Lobesia botrana. Naturwissenschaften 2008, 95, 317–324.
- Patt, J.M.; Stockton, D.; Meikle, W.G.; Sétamou, M.; Mafra-Neto, A.; Adamczyk, J.J. Innate and Conditioned Responses to Chemosensory and Visual Cues in Asian Citrus Psyllid, Diaphorina citri (Hemiptera: Liviidae), Vector of Huanglongbing Pathogens. Insects 2014, 5, 921–941.
- Behmer, S.T.; Belt, C.E.; Shapiro, M.S. Variable Rewards and Discrimination Ability in an Insect Herbivore: What and How Does a Hungry Locust Learn? J. Exp. Biol. 2005, 208, 3463–3473.
- Simoes, P.M.V.; Ott, S.R.; Niven, J.E. A Long-Latency Aversive Learning Mechanism Enables Locusts to Avoid Odours Associated with the Consequences of Ingesting Toxic Food. J. Exp. Biol. 2012, 215, 1711–1719.
- Lhomme, P.; Carrasco, D.; Larsson, M.; Hansson, B.; Anderson, P. A Context-Dependent Induction of Natal Habitat Preference in a Generalist Herbivorous Insect. Behav. Ecol. 2017, 29, 360–367.
- Zhou, D.-S.; Wang, C.-Z.; van Loon, J.J.A. Chemosensory Basis of Behavioural Plasticity in Response to Deterrent Plant Chemicals in the Larva of the Small Cabbage White Butterfly Pieris rapae. J. Insect Physiol. 2009, 55, 788–792.
- Ma, Y.; Li, J.; Tang, Q.; Zhang, X.; Zhao, X.; Yan, F.; Loon, J.J.A. van Trans-Generational Desensitization and within-Generational Resensitization of a Sucrose-Best Neuron in the Polyphagous Herbivore Helicoverpa armigera (Lepidoptera: Noctuidae). Sci. Rep. 2016, 6, 39358.
- Wada-Katsumata, A.; Silverman, J.; Schal, C. Changes in Taste Neurons Support the Emergence of an Adaptive Behavior in Cockroaches. Science 2013, 340, 972–975.
- Gadenne, C.; Barrozo, R.B.; Anton, S. Plasticity in Insect Olfaction: To Smell or Not to Smell? Annu. Rev. Entomol. 2016, 61, 317–333.
- Desouhant, E.; Driessen, G.; Amat, I.; Bernstein, C. Host and Food Searching in a Parasitic Wasp Venturia canescens: A Trade-off between Current and Future Reproduction? Anim. Behav. 2005, 70, 145–152.
- Sayin, S.; Boehm, A.C.; Kobler, J.M.; De Backer, J.-F.; Grunwald Kadow, I.C. Internal State Dependent Odor Processing and Perception—The Role of Neuromodulation in the Fly Olfactory System. Front. Cell Neurosci. 2018, 12, 11.
- Akami, M.; Andongma, A.A.; Zhengzhong, C.; Nan, J.; Khaeso, K.; Jurkevitch, E.; Niu, C.-Y.; Yuval, B. Intestinal Bacteria Modulate the Foraging Behavior of the Oriental Fruit Fly Bactrocera dorsalis (Diptera: Tephritidae). PLoS ONE 2019, 14, e0210109.
- Wong, A.C.-N.; Wang, Q.-P.; Morimoto, J.; Senior, A.M.; Lihoreau, M.; Neely, G.G.; Simpson, S.J.; Ponton, F. Gut Microbiota Modifies Olfactory-Guided Microbial Preferences and Foraging Decisions in Drosophila. Curr. Biol. 2017, 27, 2397–2404.
- Sochard, C.; Le Floch, M.; Anton, S.; Outreman, Y.; Simon, J.-C. Limited Influence of Gain and Loss of Symbionts on Host Plant Selection in Specialized Pea Aphid Genotypes. Entomologia 2021, 41, 39–47.
- Tasnin, M.S.; Merkel, K.; Clarke, A.R. Effects of Advanced Age on Olfactory Response of Male and Female Queensland Fruit Fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). J. Insect Physiol. 2020, 122, 104024.
- Devescovi, F.; Hurtado, J.; Taylor, P.W. Mating-Induced Changes in Responses of Female Queensland Fruit Fly to Male Pheromones and Fruit: A Mechanism for Mating-Induced Sexual Inhibition. J. Insect Physiol. 2021, 129, 104195.
- Nissinen, A.; Kristoffersen, L.; Anderbrant, O. Physiological State of Female and Light Intensity Affect the Host-Plant Selection of Carrot Psyllid, Trioza apicalis. Eur J Entomol 2008, 105, 227.
- Masante-Roca, I.; Anton, S.; Delbac, L.; Dufour, M.-C.; Gadenne, C. Attraction of the Grapevine Moth to Host and Non-Host Plant Parts in the Wind Tunnel: Effects of Plant Phenology, Sex, and Mating Status. Entomol. Exp. Appl. 2007, 122, 239–245.
- Landolt, P.J. Attraction of the Cabbage Looper to Host Plants and Host Plant Odor in the Laboratory. Entomol. Exp. Appl. 1989, 53, 117–123.
- Reddy, G.V.P.; Guerrero, A. Interactions of Insect Pheromones and Plant Semiochemicals. Trends Plant Sci. 2004, 9, 253–261.
- Varela, N.; Avilla, J.; Anton, S.; Gemeno, C. Synergism of Pheromone and Host-Plant Volatile Blends in the Attraction of Grapholita molesta Males. Entomol. Exp. Appl. 2011, 141, 114–122.
- Yang, Z.; Bengtsson, M.; Witzgall, P. Host Plant Volatiles Synergize Response to Sex Pheromone in Codling Moth, Cydia pomonella. J. Chem. Ecol. 2004, 30, 619–629.
- Ochieng, S.A.; Park, K.-C.; Baker, T.C. Host Plant Volatiles Synergize Responses of Sex Pheromone-Specific Olfactory Receptor Neurons in Male Helicoverpa zea. J. Comp. Physiol. A 2005, 188, 325–333.
- Ju, Q.; Guo, X.; Li, X.; Jiang, X.; Jiang, X.; Ni, W.; Qu, M. Plant Volatiles Increase Sex Pheromone Attraction of Holotrichia parallela (Coleoptera: Scarabaeoidea). J. Chem. Ecol. 2017, 43, 236–242.
- Webster, B. The Role of Olfaction in Aphid Host Location. Physiol. Entomol. 2012, 37, 10–18.
- Braendle, C.; Davis, G.K.; Brisson, J.A.; Stern, D.L. Wing Dimorphism in Aphids. Heredity 2006, 97, 192–199.
- Shambaugh, G.F.; Frazier, J.L.; Castell, A.E.M.; Coons, L.B. Antennal Sensilla of Seventeen Aphid Species (Homoptera: Aphidinae). Int. J. Insect Morphol. 1978, 7, 389–404.
- Miyazaki, M. Morphology of Aphids. In Aphids, Their Biology, Natural Enemies and Control; Minks, A., Harrewijn, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 1–25.
- Dahlin, I.; Vucetic, A.; Ninkovic, V. Changed Host Plant Volatile Emissions Induced by Chemical Interaction between Unattacked Plants Reduce Aphid Plant Acceptance with Intermorph Variation. J. Pest. Sci. 2014, 88, 249–257.
- Greenwood, M.; Chapman, R. Differences in Numbers of Sensilla on the Antennae of Solitarious and Gregarious Locusta migratoria L. (Orthoptera: Acrididae). Int. J. Insect Morphol. 1984, 13, 295–301.
- Guo, W.; Ren, D.; Zhao, L.; Jiang, F.; Song, J.; Wang, X.; Kang, L. Identification of Odorant-Binding Proteins (OBPs) and Functional Analysis of Phase-Related OBPs in the Migratory Locust. Front. Physiol. 2018, 9, 984.
- Anton, S.; Ignell, R.; Hansson, B.S. Developmental Changes in the Structure and Function of the Central Olfactory System in Gregarious and Solitary Desert Locusts. Microsc. Res. Tech. 2002, 56, 281–291.
- Ott, S.R.; Rogers, S.M. Gregarious Desert Locusts Have Substantially Larger Brains with Altered Proportions Compared with the Solitarious Phase. Proc. R. Soc. B Biol. Sci. 2010, 277, 3087–3096.
- Ignell, R.; Anton, S.; Hansson, B.S. Central Nervous Processing of Behaviourally Relevant Odours in Solitary and Gregarious Fifth Instar Locusts, Schistocerca gregaria. J. Comp. Physiol. A 1998, 183, 453–465.
- Simoes, P.M.V.; Ott, S.R.; Niven, J.E. Environmental Adaptation, Phenotypic Plasticity, and Associative Learning in Insects: The Desert Locust as a Case Study. Integr. Comp. Biol. 2016, 56, 914–924.
- Anton, S.; Jacquin-Joly, E. Médiateurs Chimiques et Lutte Contre Les Insectes. In Biocontrôle; Quae: Versailles, France, 2020; pp. 221–228. ISBN 978-2-7592-3077-8.
- Khan, Z.R.; James, D.G.; Midega, C.A.O.; Pickett, J.A. Chemical Ecology and Conservation Biological Control. Biol. Control 2008, 45, 210–224.
