An antioxidant compound can be defined as a substance that can delay or prevent oxidation. The body uses different strategies against the production and accumulation of reactive oxygen species (ROS): firstly, antioxidant enzymes are used, as already reported, such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-px). It is important to remember that some antioxidant enzymes need micronutrients to function properly, such as zinc, selenium, copper and manganese. Secondly, ROS can be reduced or neutralized by the intake of antioxidant nutrients, such as vitamin E (a-tocopherol), beta-carotene, and vitamin C, among others. An insufficient intake of foods with antioxidant function or an unbalanced diet can alter the body’s natural antioxidant system and facilitate the damage induced by ROS. Additional defense mechanisms include antioxidant compounds, such as metallothionein, melanin, and glutathione. Eye health is crucial, and the onset of diseases can reduce vision and affect the quality of life of patients. Evidence has accumulated that polyphenols (mostly deriving from Citrus Bergamia) represent a reliable source of antioxidants able to counteract oxidative stress accompanying early stages of eye diseases. Luteolin in particular has been found to protect photoreceptors, thereby improving vision in many disease states. Moreover, a consistent anti-inflammatory response was found to occur when curcumin is used alone or in combination with other nutraceuticals. Additionally, Coenzyme Q10 has been demonstrated to produce a consistent effect in reducing ocular pressure, thereby leading to protection in patients undergoing glaucoma. Both grape seed extract, rich in anthocyanosides, and polynsatured fatty acids seem to contribute to the prevention of retinal disorders. A combination of nutraceuticals and antioxidants may represent the right solution for a multi-action activity in eye protection.
- eyes diseases
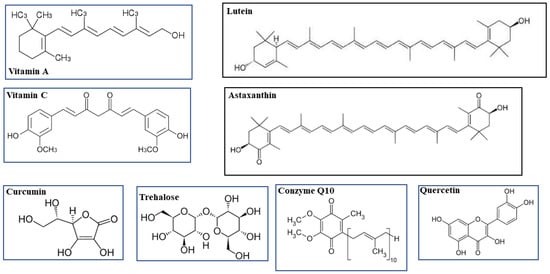
- Vitamin A
- lutein
1. Vitamin A and Lutein
2. Vitamin C and Coenzyme Q10
3. Astaxanthin
5. Curcumin and Quercetin
6. PUFAs
7. Grape Seed Extract and Bergamot Polyphenolic Fraction
References
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703.
- Saari, J.C. Vitamin A and Vision. Subcell Biochem. 2016, 81, 231–259.
- Koekkoek, W.A.; van Zanten, A.R. Antioxidant Vitamins and Trace Elements in Critical Illness. Nutr. Clin. Pract. 2016, 31, 457–474.
- Blaner, W.S.; Shmarakov, I.O.; Traber, M.G. Vitamin A and Vitamin E: Will the Real Antioxidant Please Stand Up? Annu. Rev. Nutr. 2021, 41, 105–131.
- Bartlett, H.; Eperjesi, F. An ideal ocular nutritional supplement? Ophthal. Physiol. Opt. 2004, 24, 339–349.
- Martini, D.; Negrini, L.; Marino, M.; Riso, P.; Del Bo, C.; Porrini, M. What Is the Current Direction of the Research on Carotenoids and Human Health? An Overview of Registered Clinical Trials. Nutrients 2022, 14, 1191.
- Perry, A.; Rasmussen, H.; Johnson, E. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15.
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66.
- Li, B.; George, E.W.; Rognon, G.T.; Gorusupudi, A.; Ranganathan, A.; Chang, F.Y.; Shi, L.; Frederick, J.M.; Bernstein, P.S. Imaging lutein and zeaxanthin in the human retina with confocal resonance Raman microscopy. Proc. Natl. Acad. Sci. USA 2020, 117, 12352–12358.
- Manayi, A.; Abdollahi, M.; Raman, T.; Nabavi, S.F.; Habtemariam, S.; Daglia, M.; Nabavi, S.M. Lutein and cataract: From bench to bedside. Crit. Rev. Biotechnol. 2016, 36, 829–839.
- Yoshizako, H.; Hara, K.; Takai, Y.; Kaidzu, S.; Obana, A.; Ohira, A. Comparison of macular pigment and serum lutein concentration changes between free lutein and lutein esters supplements in Japanese subjects. Acta Ophthalmol. 2016, 94, e411–e416.
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T. Lutein: More than just a filter for blue light. Prog. Retin. Eye Res. 2012, 31, 303–315.
- Junghans, A.; Sies, H.; Stahl, W. Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch. Biochem. Biophys. 2001, 391, 160–164.
- Rafi, M.M.; Shafaie, Y. Dietary lutein modulates inducible nitric oxide synthase (iNOS) gene and protein expression in mouse macrophage cells (RAW 264.7). Mol. Nutr. Food Res. 2007, 51, 333–340.
- Chung, R.W.S.; Leanderson, P.; Lundberg, A.K.; Jonasson, L. Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis 2017, 262, 87–93.
- Liu, T.; Liu, W.H.; Zhao, J.S.; Meng, F.Z.; Wang, H. Lutein protects against β-amyloid peptide-induced oxidative stress in cerebrovascular endothelial cells through modulation of Nrf-2 and NFkB. Cell. Biol. Toxicol. 2017, 33, 57–67.
- Chang, J.; Zhang, Y.; Li, Y.; Lu, K.; Shen, Y.; Guo, Y.; Qi, Q.; Wang, M.; Zhang, S. NrF2/ARE and NF-kB pathway regulation may be the mechanism for lutein inhibition of human breast cancer cell. Future Oncol. 2018, 14, 719–726.
- Tian, Y.; Kijlstra, A.; van der Veen, R.L.; Makridaki, M.; Murray, I.J.; Berendschot, T.T. Lutein supplementation leads to decreased soluble complement membrane attack complex sC5b-9 plasma levels. Acta Ophthalmol. 2015, 93, 141–145.
- Li, L.H.; Lee, J.C.-Y.; Leung, H.H.; Lam, W.C.; Fu, Z.; Lo, A.C.Y. Lutein Supplementation for Eye Diseases. Nutrients 2020, 12, 1721.
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.L.; Satriano, A.; Marchesini, G. The Effect of Lutein on Eye and Extra-Eye Health. Nutrients 2018, 10, 1321.
- Ranard, K.M.; Jeon, S.; Mohn, E.S.; Griffiths, J.C.; Johnson, E.J.; Erdman, J.W., Jr. Dietary guidance for lutein: Consideration for intake recommendations is scientifically supported. Eur. J. Nutr. 2017, 56 (Suppl. 3), 37–42.
- Stahl, W. Macular carotenoids: Lutein and zeaxanthin. Dev. Ophthalmol. 2005, 38, 70–88.
- Food and Nutrition Board Staff, Panel on Dietary Antioxidants; Institute of Medicine Staff. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academy of Sciences: Washington, DC, USA, 2000.
- Shao, A.; Hathcock, J.N. Risk assessment for the carotenoids lutein and lycopene. Regul. Toxicol. Pharm. 2006, 45, 289–298.
- Pehlivan, F.E. Vitamin C-an antioxidant agent. In Vitamin C; Hamza, A.H., Ed.; IntechOpen: London, UK, 2017.
- Koppenol, W.H.; Hider, R.H. Iron and Redox Cycling. Do’s and Don’ts. Free Radic. Biol. Med. 2019, 133, 3–10.
- Shui, Y.B.; Holekamp, N.M.; Kramer, B.C.; Crowley, J.R.; Wilkins, M.A.; Chu, F.; Malone, P.E.; Mangers, S.J.; Hou, J.H.; Siegfried, C.J.; et al. The gel state of the vitreous and ascorbate-dependent oxygen consumption: Relationship to the etiology of nuclear cataracts. Arch. Ophthalmol. 2009, 127, 475–482.
- Barros, A.I.R.N.A.; Nunes, F.M.; Gonçalves, B.; Bennett, R.N.; Silva, A.P. Effect of cooking on total vitamin C contents and antioxidant activity of sweet chestnuts. Food Chem. 2011, 128, 165–172.
- Brubaker, R.F.; Bourne, W.M.; Bachman, L.A.; McLaren, J.W. Ascorbic acid content of human corneal epithelium. Investig. Opthalmol. Vis. Sci. 2000, 41, 1681–1683.
- Talluri, R.S.; Katragadda, S.; Pal, D.; Mitra, A.K. Mechanism of Lascorbic acid uptake by rabbit corneal epithelial cells: Evidence for the involvement of sodium-dependent vitamin C transporter 2. Curr. Eye Res. 2006, 31, 481–489.
- Liu, F.; Xiong, J.; Hu, J.; Ran, Z.; Wang, J.; Li, Z.; Chen, M.; Wang, Y. Vitamin C and risk of age-related cataracts: A systematic review and meta-analysis. Int. J. Clin. Exp. Med. 2018, 11, 8929–8940.
- Wei, L.; Liang, G.; Cai, C.; Lv, J. Association of vitamin C with the risk of age-related cataract: A meta-analysis. Acta Ophthalmol. 2016, 94, e170–e176.
- Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academy of Sciences Press: Washington, DC, USA, 2006.
- Frei, B.; Birlouez, I.; Lykkesfeldt, J. What is the optimum intake of vitamin C in humans? Crit. Rev. Food Sci. Nutr. 2012, 52, 815–829.
- Bhagavan, H.N.; Chopra, R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic. Res. 2006, 40, 445–453.
- Acosta, M.J.; Fonseca, L.V.; Desbats, M.A.; Cerqua, C.; Zordan, R.; Trevisson, E.; Salviati, L. Coenzyme Q biosynthesis in health and disease. Biochim. Biophy. Acta (BBA)-Bioenerg. 2016, 1857, 1079–1085.
- Manzar, H.; Abdulhussein, D.; Yap, T.E.; Cordeiro, M.F. Cellular Consequences of Coenzyme Q10 Deficiency in Neurodegeneration of the Retina and Brain. Int. J. Mol. Sci. 2020, 21, 9299.
- Hargreaves, I.; Heaton, R.A.; Mantle, D. Disorders of Human Coenzyme Q10 Metabolism: An Overview. Int. J. Mol. Sci. 2020, 21, 6695.
- Groneberg, D.A.; Kindermann, B.; Althammer, M.; Klapper, M.; Vormann, J.; Littarru, J.; Doring, F. Coenzyme Q10 affects expression of genes involved in cell signalling, metabolism and transport in human CaCo-2 cells. Int. J. Biochem. Cell Biol. 2005, 37, 1208–1218.
- Tsui, H.S.; Pham, N.V.B.; Amer, B.R.; Bradley, M.C.; Gosschalk, J.E.; Gallagher-Jones, M.; Ibarra, H.; Clubb, R.T.; Blaby-Haas, C.E.; Clarke, C.F. Human COQ10A and COQ10B are distinct lipid-binding START domain proteins required for coenzyme Q function. J. Lipid Res. 2019, 60, 1293–1310.
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. Coenzyme Q10 supplementation: Efficacy, safety, and formulation challenges. Compr. Rev. Food Sci. Food Saf. 2020, 19, 574–594.
- Awad, A.M.; Bradley, M.C.; Fernández-Del-Río, L.; Nag, A.; Tsui, H.S.; Clarke, C.F. Coenzyme Q10 deficiencies: Pathways in yeast and humans. Essays Biochem. 2018, 62, 361–376.
- Quinzii, C.M.; López, L.C.; Gilkerson, R.W.; Dorado, B.; Coku, J.; Naini, A.B.; Lagier-Tourenne, C.; Schuelke, M.; Salviati, L.; Carrozzo, R.; et al. Reactive oxygen species, oxidative stress, and cell death correlate with level of CoQ10 deficiency. FASEB J. 2010, 24, 3733–3743.
- Quinzii, C.M.; Luna-Sanchez, M.; Ziosi, M.; Hidalgo-Gutierrez, A.; Kleiner, G.; Lopez, L.C. The role of sulfide oxidation impairment in the pathogenesis of primary CoQ deficiency. Front. Physiol. 2017, 8, 525.
- Heaton, R.A.; Heales, S.; Rahman, K.; Sexton, D.W.; Hargreaves, I. The Effect of Cellular Coenzyme Q10 Deficiency on Lysosomal Acidification. J. Clin. Med. 2020, 9, 1923.
- Rötig, A.; Appelkvist, E.L.; Geromel, V.; Chretien, D.; Kadhom, N.; Edery, P.; Lebideau, M.; Dallner, G.; Munnich, A.; Ernster, L.; et al. Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet 2000, 356, 391–395.
- Mollet, J.; Giurgea, I.; Schlemmer, D.; Dallner, G.; Chretien, D.; Delahodde, A.; Bacq, D.; de Lonlay, P.; Munnich, A.; Rötig, A. Prenyldiphosphate synthase, subunit 1 (PDSS1) and OH-benzoate polyprenyltransferase (COQ2) mutations in ubiquinone deficiency and oxidative phosphorylation disorders. J. Clin. Investig. 2007, 117, 765–772.
- Qu, J.; Kaufman, Y.; Washington, I. Coenzyme Q10 in the human retina. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1814–1818.
- Dusting, G.J.; Triggle, C. Are we over oxidized? Oxidative stress, cardiovascular disease, and the future of intervention studies with antioxidants. Vasc. Health Risk Manag. 2005, 1, 93–97.
- Nunomura, A.; Moreira, P.I.; Lee, H.G.; Zhu, X.; Castellani, R.J.; Smith, M.A.; Perry, G. Neuronal Death and Survival Under Oxidative Stress in Alzheimer and Parkinson Diseases. CNS Neurol. Disord. Drug Targets 2008, 6, 411–423.
- Lenaz, G. Role of mitochondria in oxidative stress and ageing. Biochim. Biophys. Acta Bioenerg. 1998, 1366, 53–67.
- Sas, K.; Szabó, E.; Vécsei, L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules 2018, 23, 191.
- Bilbao-Malavé, V.; González-Zamora, J.; de la Puente, M.; Recalde, S.; Fernandez-Robredo, P.; Hernandez, M.; Layana, A.G.; Saenz de Viteri, M. Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in Age Related Macular Degeneration, Role in Pathophysiology, and Possible New Therapeutic Strategies. Antioxidants 2021, 10, 1170.
- Schniertshauer, D.; Gebhard, D.; Bergemann, J. Age-Dependent Loss of Mitochondrial Function in Epithelial Tissue Can Be Reversed by Coenzyme Q10. J Aging Res. 2018, 2018, 6354680.
- Lee, D.; Shim, M.S.; Kim, K.Y.; Noh, Y.H.; Kim, H.; Kim, S.Y.; Weinreb, R.N.; Ju, W.K. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress–mediated mitochondrial alteration in a mouse model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 993–1005.
- Miles, M.V. The uptake and distribution of coenzyme Q10. Mitochondrion 2007, 7, S72–S77.
- Nucci, C.; Tartaglione, R.; Cerulli, A.; Mancino, R.; Spano, A.; Cavaliere, F.; Rombola, L.; Bagetta, G.; Corasaniti, M.T.; Morrone, L.A. Retinal damage caused by high intraocular pressure–induced transient ischemia is prevented by coenzyme Q10 in rat. Int. Rev. Neurobiol. 2007, 82, 397–406.
- Russo, R.; Cavaliere, F.; Rombolà, L.; Gliozzi, M.; Cerulli, A.; Nucci, C.; Fazzi, E.; Bagetta, G.; Corasaniti, M.T.; Morrone, L.A. Rational basis for the development of coenzyme Q10 as a neurotherapeutic agent for retinal protection. Prog. Brain Res. 2008, 173, 575–582.
- Mancini, A.; Festa, R.; Raimondo, S.; Pontecorvi, A.; Littarru, G.P. Hormonal influence on coenzyme Q10 levels in blood plasma. Int. J. Mol. Sci. 2011, 12, 9216–9225.
- Shishodia, S. Molecular mechanisms of curcumin action: Gene expression. Biofactors 2013, 39, 37–55.
- Mordi, R.C.; Ademosun, O.T.; Ajanaku, C.O.; Olanrewaju, I.O.; Walton, J.C. Free Radical Mediated Oxidative Degradation of Carotenes and Xanthophylls. Molecules 2020, 25, 1038.
- Moukarzel, A.A.; Bejjani, R.A.; Fares, F.N. Xanthophylls and eye health of infants and adults. J. Med. Liban. 2009, 57, 261–267.
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165.
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. 2011, 16, 355–364.
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20.
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152.
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522.
- Giannaccare, G.; Pellegrini, M.; Senni, C.; Bernabei, F.; Scorcia, V.; Cicero, A.F.G. Clinical Applications of Astaxanthin in the Treatment of Ocular Diseases: Emerging Insights. Mar. Drugs 2020, 18, 239.
- Nishida, Y.; Nawaz, A.; Hecht, K.; Tobe, K. Astaxanthin as a Novel Mitochondrial Regulator: A New Aspect of Carotenoids, beyond Antioxidants. Nutrients 2021, 14, 107.
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196.
- McNulty, H.P.; Byun, J.; Lockwood, S.F.; Jacob, R.F.; Mason, R.P. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Biophys. Acta 2007, 1768, 167–174.
- Choi, H.D.; Kim, J.H.; Chang, M.J.; Kyu-Youn, Y.; Shin, W.G. Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytother. Res. 2011, 25, 1813–1818.
- Grattagliano, I.; Palmieri, V.O.; Portincasa, P.; Moschetta, A.; Palasciano, G. Oxidative stress-induced risk factors associated with the metabolic syndrome: A unifying hypothesis. J. Nutr. Biochem. 2008, 19, 491–504.
- Kim, J.H.; Chang, M.J.; Choi, H.D. Protective effects of Haematococcus astaxanthin on oxidative stress in healthy smokers. J. Med. Food 2011, 14, 1469–1475.
- Xue, X.L.; Han, X.D.; Li, Y.; Chu, X.F.; Miao, W.M.; Zhang, J.L.; Fan, S.J. Astaxanthin attenuates total body irradiation-induced hematopoietic system injury in mice via inhibition of oxidative stress and apoptosis. Stem Cell Res. Ther. 2017, 8, 7.
- Fang, Q.; Guo, S.; Zhou, H.; Han, R.; Wu, P.; Han, C. Astaxanthin protects against early burn-wound progression in rats by attenuating oxidative stress-induced inflammation and mitochondria-related apoptosis. Sci. Rep. 2017, 7, 41440.
- Macedo, R.C.; Bolin, A.P.; Marin, D.P.; Otton, R. Astaxanthin addition improves human neutrophils function: In vitro study. Eur. J. Nutr. 2010, 49, 447–457.
- De la Fuente, M. Effects of antioxidants on immune system ageing. Eur. J. Clin. Nutr. 2002, 56, S5–S8.
- Yamagishi, R.; Aihara, M. Neuroprotective effect of astaxanthin against rat retinal ganglion cell death under various stresses that induce apoptosis and necrosis. Mol. Vis. 2014, 20, 1796–1805.
- Otsuka, T.; Shimazawa, M.; Nakanishi, T.; Ohno, Y.; Inoue, Y.; Tsuruma, K.; Ishibashi, T.; Hara, H. Protective effects of a dietary carotenoid, astaxanthin, against light-induced retinal damage. J. Pharmacol. Sci. 2013, 123, 209–218.
- Libkind, D.; Moliné, M.; Colabella, F. Isolation and Selection of New Astaxanthin-Producing Strains of Phaffia rhodozyma. Methods Mol Biol. 2018, 1852, 297–310.
- O’Neill, M.K.; Piligian, B.F.; Olson, C.D.; Woodruff, P.J.; Swarts, B.M. Tailoring Trehalose for Biomedical and Biotechnological Applications. Pure Appl. Chem. 2017, 89, 1223–1249.
- Hosseinpour-Moghaddam, K.; Caraglia, M.; Sahebkar, A. Autophagy induction by trehalose: Molecular mechanisms and therapeutic impacts. J. Cell. Physiol. 2018, 233, 6524–6543.
- Stewart, S.; He, X. Intracellular Delivery of Trehalose for Cell Banking. Langmuir 2018, 35, 7414–7422.
- Zhang, M.; Oldenhof, H.; Sieme, H.; Wolkers, W.F. Combining endocytic and freezing-induced trehalose uptake for cryopreservation of mammalian cells. Biotechnol. Prog. 2017, 33, 229–235.
- Menzies, F.M.; Fleming, A.; Caricasole, A.; Bento, C.F.; Andrews, S.P.; Ashkenazi, A.; Füllgrabe, J.; Jackson, A.; Jimenez Sanchez, M.; Karabiyik, C.; et al. Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron 2017, 93, 1015–1034.
- Takahashi, S.; Isaka, M.; Hamaishi, M.; Imai, K.; Orihashi, K.; Sueda, T. Trehalose protects against spinal cord ischemia in rabbits. J. Vasc. Surg. 2014, 60, 490–496.
- Portbury, S.D.; Hare, D.J.; Finkelstein, D.I.; Adlard, P.A. Trehalose improves traumatic brain injury-induced cognitive impairment. PLoS ONE 2017, 12, e0183683.
- Maiuolo, J.; Macrì, R.; Bava, I.; Gliozzi, M.; Musolino, V.; Nucera, S.; Carresi, C.; Scicchitano, M.; Bosco, F.; Scarano, F.; et al. Myelin Disturbances Produced by Sub-Toxic Concentration of Heavy Metals: The Role of Oligodendrocyte Dysfunction. Int. J. Mol. Sci. 2019, 20, 4554.
- Meyer, N.; Rinholm, J.E. Mitochondria in Myelinating Oligodendrocytes: Slow and Out of Breath. Metabolites 2021, 11, 359.
- Ugarte, M.; Osborne, N.N. Recent advances in the understanding of the role of zinc in ocular tissues. Metallomics 2014, 6, 189–200.
- Sarkar, S.; Davies, J.E.; Huang, Z.; Tunnacliffe, A.; Rubinsztein, D.C. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J. Biol. Chem. 2007, 282, 5641–5652.
- Zhang, Y.; DeBosch, B.J. Using trehalose to prevent and treat metabolic function: Effectiveness and mechanisms. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 303–310.
- Kaushik, J.K.; Bhat, R. Why trehalose an exceptional protein stabilizer? An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose. J. Biol. Chem. 2003, 278, 26458–26465.
- Jain, N.K.; Roy, I. Trehalose and protein stability. Curr. Protoc. Protein Sci. 2010, 59, 4–9.
- Hill-Bator, A.; Misiuk-Hojto, M.; Marycz, K.; Grzesiak, J. Trehalose-based eye drops preserve viability and functionality of cultured human corneal epithelial cells during desiccation. Biomed. Res. Int. 2014, 2014, 292139.
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroli, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R.
- Laihia, J.; Kaarniranta, K. Trehalose for Ocular Surface Health. Biomolecules 2020, 10, 809.
- Lee, H.J.; Yoon, Y.S.; Lee, S.J. Mechanism of neuroprotection by trehalose: Controversy surrounding autophagy induction. Cell Death Dis. 2018, 9, 712.
- Chiambaretta, F.; Doan, S.; Labetoulle, M.; Rocher, N.; Fekin, L.E.; Messaoud, R.; Khairallah, M.; Baudouin, C. A randomized, controlled study of the efficacy and safety of new eyedrop formulation for moderate to severe dry eye. Eur. J. Ophthalmol. 2017, 27, 1–9.
- Lievens, C.; Berdy, G.; Douglass, D.; Montaquila, S.; Lin, H.; Simmons, P.; Carlisle-Wilcox, C.; Vehige, J.; Haque, S. Evaluation of an enhanced viscosity artificial tear for moderate to severe dry eye disease: A multicenter, double-masked, randomized 30-day study. Cont Lens Anterior Eye. 2019, 42, 443–449.
- Pinto-Bonilla, J.C.; Del Olmo-Jimeno, A.; Llovet-Osuna, F.; Hernander-Gallilea, E. A randomized crossover study comparing trehalose/hyaluronate eyedrops and standard treatment:patient satisfaction in the treatment of dry eye syndrome. Ther. Clin. Risk Manag. 2015, 11, 595–603.
- Čejková, J.; Stipek, S.; Crkovska, J.; Ardan, T.; Platenik, J.; Cejka, C.; Midelfart, A. UV Rays, the prooxidant/antioxidant imbalance in the cornea and oxidative damage. Physiol. Res. 2004, 53, 1–10.
- Čejková, J.; Cejka, C.; Luyckx, J. Trehalose treatment accelerates the healing of UVB-irradiated corneas. Comparative immunohistochemical studies on corneal cryostat sections and corneal impression cytology. Histol. Histopathol. 2012, 27, 1029–1040.
- Talero, E.; Ávila-Roman, J.; Motilva, V. Chemoprevention with phytonutrients and microalgae products in chronic inflammation and colon cancer. Curr. Pharm Des. 2012, 18, 3939–3965.
- Taylor, R.A.; Leonard, M.C. Curcumin for inflammatory bowel disease: A review of human studies. Altern. Med. Rev. 2011, 16, 152–156.
- Carmona-Ramírez, I.; Santamaría, A.; Tobón-Velasco, J.C.; Orozco-Ibarra, M.; González-Herrera, I.G.; Pedraza-Chaverrí, J.; Maldonado, P.D. Curcumin restores Nrf2 levels and prevents quinolinic acid-induced neurotoxicity. J. Nutr. Biochem. 2013, 24, 14–24.
- Aggarwal, S.; Ichikawa, H.; Takada, Y.; Sandur, S.K.; Shishodia, S.; Aggarwal, B.B. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IκBα Kinase and akt activation. Mol. Pharmacol. 2006, 69, 195–206.
- Prudʼhomme, G.J. Cancer stem cells and novel targets for antitumor strategies. Curr. Pharm. Des. 2012, 18, 2838–2849.
- Radomska-Leśniewska, D.M.; Osiecka-Iwan, A.; Hyc, A.; Góźdź, A.; Dąbrowska, A.M.; Skopiński, P. Therapeutic potential of curcumin in eye diseases. Cent. Eur. J. Immunol. 2019, 44, 181–189.
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125.
- Lin, Y.G.; Kunnumakkara, A.B.; Nair, A.; Merritt, W.M.; Han, L.Y.; Armaiz-Pena, G.N.; Kamat, A.A.; Spannuth, W.A.; Gershenson, D.M.; Lutgendorf, S.K.; et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-κB pathway. Clin. Cancer Res. 2007, 13, 3423–3430.
- Marchiani, A.; Rozzo, C.; Fadda, A.; Delogu, G.; Ruzza, P. Curcumin and curcumin-like molecules: From spice to drugs. Curr. Med. Chem. 2014, 21, 204–222.
- Ushio-Fukai, M.; Nakamura, Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008, 266, 37–52.
- Ushio-Fukai, M. Redox signaling in angiogenesis: Role of NADPH oxidase. Cardiovasc Res. 2006, 71, 226–235.
- Radomska-Leśniewska, D.M.; Skopiński, P.; Bałan, J.B.; Białoszewska, A.; Jóźwiak, J.; Rokicki, D.; Skopińska-Różewska, E.; Borecka, A.; Hevelke, A. Angiomodulatory properties of Rhodiola spp. And other natural antioxidants. Cent. Eur. J. Immunol. 2015, 40, 249–262.
- Radomska-Leśniewska, D.M.; Bałan, J.B.; Skopiński, P. Angiogenesis modulation by exogenous antioxidants. Cent. Eur. J. Immunol. 2017, 42, 370–376.
- Nebbioso, M.; Franzone, F.; Greco, A.; Gharbiya, M.; Bonofiglio, V.; Polimeni, A. Recent Advances and Disputes About Curcumin in Retinal Diseases. Clin. Ophthalmol. 2021, 15, 2553–2571.
- Munia, I.; Gafray, L.; Bringer, M.A.; Goldschmidt, P.; Proukhnitzky, L.; Jacquemot, N.; Cercy, C.; Ramchani Ben Otman, K.; Errera, M.H.; Ranchon-Cole, I. Cytoprotective effects of natural highly bio-available vegetable derivatives on human-derived retinal cells. Nutrients 2020, 12, 879.
- Niederkorn, J.Y.; Stern, M.E.; Pflugfelder, S.C.; De Paiva, C.S.; Corrales, R.M.; Gao, J.; Siemasko, K. Desiccating stress induces T cell-mediated Sjogren’s Syndrome-like lacrimal keratoconjunctivitis. J. Immunol. 2006, 176, 3950–3957.
- Li, D.Q.; Luo, L.; Chen, Z.; Li, D.-Q.; Kim, H.-S.; Song, X.J.; Pflugfelder, S.C. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp. Eye Res. 2006, 82, 588–596.
- Chung, S.H.; Choi, S.H.; Choi, J.A.; Chuck, R.S.; Joo, C.K. Curcumin suppresses ovalbumin-induced allergic conjunctivitis. Mol. Vis. 2012, 18, 1966–1972.
- Gupta, S.K.; Agarwal, R.; Srivastava, S.; Agarwal, P.; Agrawal, S.U.; Saxena, R.; Galpalli, N. The anti-inflammatory effects of Curcuma longa and Berberis aristata in endotoxin-induced uveitis in rabbits. Investig. Ophthalmol. Vis. Sci. 2008, 4, 4036–4040.
- Agarwal, R.; Gupta, S.K.; Agarwal, P.; Srivastava, S. Topically applied standardized aqueous extract of Curcuma longa Linn. suppresses endotoxin-induced uveal inflammation in rats. Indian J. Exp. Biol. 2013, 51, 797–803.
- Michalik, L.; Auwerx, J.; Berger, J.P.; Chatterjee, V.K.; Glass, C.K.; Gonzalez, F.J.; Grimaldi, P.A.; Kadowaki, T.; Lazar, M.A.; O’Rahilly, S.; et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 2006, 58, 726–741.
- Salehi, M.; Mashhadi, N.S.; Esfahani, P.S.; Feizi, A.; Hadi, A.; Askari, G. The Effects of Curcumin Supplementation on Muscle Damage, Oxidative Stress, and Inflammatory Markers in Healthy Females with Moderate Physical Activity: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Int. J. Prev. Med. 2021, 12, 94.
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123.
- Tang, S.M.; Deng, X.T.; Zhou, J.; Li, Q.P.; Ge, X.X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604.
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177.
- Capriglione, F.; Maiuolo, J.; Celano, M.; Damante, G.; Russo, D.; Bulotta, S.; Maggisano, V. Quercetin Protects Human Thyroid Cells against Cadmium Toxicity. Int. J. Mol. Sci. 2021, 22, 6849.
- Shen, C.Y.; Jiang, J.G.; Yan, G.L.; Wang, D.W.; Zhu, W. Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: Pharmacological mechanisms and implications for drug discovery. Br. J. Pharmacol. 2017, 174, 1395–1425.
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Huang, Y.; Tu, S. Potential Implications of Quercetin in Autoimmune Diseases. Front. Immunol. 2021, 12, 689044.
- Yi, H.; Peng, H.; Wu, X.; Xu, X.; Kuang, T.; Zhang, J.; Fan, G. The Therapeutic Effects and Mechanisms of Quercetin on Metabolic Diseases: Pharmacological Data and Clinical Evidence. Oxid. Med. Cell Longev. 2021, 2021, 6678662.
- McKay, T.B.; Karamichos, D. Quercetin and the ocular surface: What we know and where we are going. Exp. Biol. Med. 2017, 242, 565–572.
- Liu, Y.; Gan, L.; Carlsson, D.J.; Fagerholm, P.; Lagali, N.; Watsky, M.A.; Munger, R.; Hodge, W.G.; Priest, D.; Griffith, M. A simple, cross-linked collagen tissue substitute for corneal implantation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1869–1875.
- Davies, N.M. Biopharmaceutical considerations in topical ocular drug delivery. Clin. Exp. Pharmacol. Physiol. 2000, 27, 558–562.
- Nishimuro, H.; Ohnishi, H.; Sato, M.; Ohnishi-Kameyama, M.; Matsunaga, I.; Naito, S.; Ippoushi, K.; Akasaka, H.; Saitoh, S.; Shimamoto, K.; et al. Estimated daily intake and seasonal food sources of quercetin in Japan. Nutrients 2015, 7, 2345–2358.
- Neamtu, A.A.; Maghiar, T.A.; Alaya, A.; Olah, N.K.; Turcus, V.; Neamtu, C.; Maghiar, A.M.; Mathe, E. A Comprehensive View on the Quercetin Impact on Colorectal Cancer. Molecules 2022, 27, 1873.
- Oppedisano, F.; Bulotta, R.M.; Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Ilari, S.; Serra, M.; Muscoli, C.; Gratteri, S.; et al. The Role of Nutraceuticals in Osteoarthritis Prevention and Treatment: Focus on n-3 PUFAs. Oxid. Med. Cell Longev. 2021, 2021, 4878562.
- Mollace, V.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F. Re-assessing the mechanism of action of n-3 PUFAs. Int. J. Cardiol. 2013, 170 (Suppl. 1), S8–S11.
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306.
- Oppedisano, F.; Mollace, R.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Macrì, R.; Carresi, C.; Maiuolo, J.; Serra, M.; Cardamone, A.; et al. PUFA Supplementation and Heart Failure: Effects on Fibrosis and Cardiac Remodeling. Nutrients 2021, 13, 2965.
- Oppedisano, F.; Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Scicchitano, M.; Scarano, F.; Bosco, F.; Macrì, R.; et al. The Potential for Natural Antioxidant Supplementation in the Early Stages of Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 2618.
- Gong, Y.; Fu, Z.; Liegl, R.; Chen, J.; Hellström, A.; Smith, L.E. ω-3 and ω-6 long-chain PUFAs and their enzymatic metabolites in neovascular eye diseases. Am. J. Clin. Nutr. 2017, 106, 16–26.
- Kalogerou, M.; Kolovos, P.; Prokopiou, E.; Papagregoriou, G.; Deltas, C.; Malas, S.; Georgiou, T. Omega-3 fatty acids protect retinal neurons in the DBA/2J hereditary glaucoma mouse model. Exp. Eye Res. 2018, 167, 128–139.
- Bao, J.; Yang, Z.; Zheng, S.; Li, J.; Shentu, X. Circulating fatty acids and risk of primary open-angle glaucoma: A mendelian randomization study. Gene 2022, 811, 146078.
- Romeo Villadóniga, S.; Rodríguez García, E.; Sagastagoia Epelde, O.; Álvarez Díaz, M.D.; Domingo Pedrol, J.C. Effects of Oral Supplementation with Docosahexaenoic Acid (DHA) plus Antioxidants in Pseudoexfoliative Glaucoma: A 6-Month Open-Label Randomized Trial. J. Ophthalmol. 2018, 2018, 8259371.
- Da Costa Morato Nery, D.; da Silva, C.G.; Mariani, D.; Fernandes, P.N.; Pereira, M.D.; Panek, A.D.; Eleutherio, E.C. The role of trehalose and its transporter in protection against reactive oxygen species. Biochim. Biophys. Acta 2008, 1780, 1408–1411.
- Saccà, S.C.; Cutolo, C.A.; Ferrari, D.; Corazza, P.; Traverso, C.E. The Eye, Oxidative Damage and Polyunsaturated Fatty Acids. Nutrients 2018, 10, 668.
- Padmanabha, S.; Vallikannan, B. Fatty acids modulate the efficacy of lutein in cataract prevention: Assessment of oxidative and inflammatory parameters in rats. Biochem. Biophys. Res. Commun. 2018, 500, 435–442.
- Padmanabha, S.; Vallikannan, B. Fatty acids influence the efficacy of lutein in the modulation of α-crystallin chaperone function: Evidence from selenite induced cataract rat model. Biochem. Biophys. Res. Commun. 2020, 529, 425–431.
- Monagas, M.; Quintanilla-López, J.E.; Gómez-Cordovés, C.; Bartolomé, B.; Lebrón-Aguilar, R. MALDI-TOF MS analysis of plant proanthocyanidins. J. Pharm Biomed. Anal. 2010, 51, 358–372.
- Prasain, J.K.; Peng, N.; Dai, Y.; Moore, R.; Arabshahi, A.; Wilson, L. Liquid chromatography tandem mass spectrometry identification of proanthocyanidins in rat plasma after oral administration of grape seed extract. Phytomedicine 2009, 16, 233–243.
- Gao, Z.; Liu, G.; Hu, Z.; Shi, W.; Chen, B.; Zou, P. Grape seed proanthocyanidins protect against streptozotocin-induced diabetic nephropathy by attenuating endoplasmic reticulum stress-induced apoptosis. Mol. Med. Rep. 2018, 18, 1447–1454.
- Décordé, K.; Teissèdre, P.L.; Sutra, T.; Ventura, E.; Cristol, J.P.; Rouanet, J.M. Chardonnay grape seed procyanidin extract supplementation prevents high-fat diet-induced obesity in hamsters by improving adipokine imbalance and oxidative stress markers. Mol. Nutr. Food Res. 2009, 53, 659–666.
- Chacón, M.R.; Ceperuelo-Mallafré, V.; Maymó-Masip, E.; Mateo-Sanz, J.M.; Arola, L.; Guitiérrez, C. Grape-seed procyanidins modulate inflammation on human differentiated adipocytes in vitro. Cytokine 2009, 47, 137–142.
- Hao, J.P.; Shi, H.; Zhang, J.; Zhang, C.M.; Feng, Y.M.; Qie, L.Y. Role of GSPE in improving early cerebral vascular damage by inhibition of Profilin-1 expression in a ouabain-induced hypertension model. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6999–7012.
- Xianchu, L.; Ming, L.; Xiangbin, L.; Lan, Z. Grape seed proanthocyanidin extract supplementation affects exhaustive exercise-induced fatigue in mice. Food Nutr. Res. 2018, 62, 62.
- Yang, D.; Li, S.; Gao, L.; Lv, Z.; Bing, Q.; Lv, Q. Dietary grape seed procyanidin extract protects against leadinduced heart injury in rats involving endoplasmic reticulum stress inhibition and AKT activation. J. Nutr. Biochem. 2018, 62, 43–49.
- Pons, Z.; Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Chronic administration of grape-seed polyphenols attenuates the development of hypertension and improves other cardiometabolic risk factors associated with the metabolic syndrome in cafeteria diet-fed rats. Br. J. Nutr. 2017, 117, 200–208.
- Pinent, M.; Bladé, C.; Salvadó, M.J.; Blay, M.; Pujadas, G.; Fernández-Larrea, J. Procyanidin effects on adipocyterelated pathologies. Crit. Rev. Food Sci. Nutr. 2006, 46, 543–550.
- Jhun, J.Y.; Moon, S.J.; Yoon, B.Y.; Byun, J.K.; Kim, E.K.; Yang, E.J. Grape seed proanthocyanidin extract-mediated regulation of STAT3 proteins contributes to Treg differentiation and attenuates inflammation in a murine model of obesity-associated arthritis. PLoS ONE 2013, 8, e78843.
- Sherif, A.A.; Abdelhalim, S.Z.; Salim, E.I. Immunohistochemical and biochemical alterations following administration of proanthocyanidin extract in rats hepatocellular carcinoma. Biomed. Pharmacother. 2017, 93, 1310–1319.
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Das, D.K.; Ray, S.D.; Kuszynski, C.A. Free radicals and grape seed proanthocyanidin extract: Importance in human health and disease prevention. Toxicology 2000, 148, 187–197.
- Mollace, V.; Rosano, G.M.C.; Anker, S.D.; Coats, A.J.S.; Seferovic, P.; Mollace, R.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Carresi, C.; et al. Pathophysiological Basis for Nutraceutical Supplementation in Heart Failure: A Comprehensive Review. Nutrients 2021, 13, 257.
- Rodríguez-Pérez, C.; García-Villanova, B.; Guerra-Hernández, E.; Verardo, V. Grape Seeds proanthocyanidins: An overview of in vivo bioactivity in animal models. Nutrients 2019, 11, 2435.
