Inflammatory bowel diseases are characterized also by retarded growth and delayed puberty. The underlying mechanism of these and other extra-intestinal manifestations are partially known: the main hypotheses are malnutrition and inflammatory response.
- growth failure
- delayed puberty
- ulcerative colitis
- Crohn’s disease
1.
Inflammatory bowel diseases (IBD) are gastrointestinal tract pathologies of unknown etiology; they have an alternating trend, with active and silent phases. IBD are classified in two main forms: ulcerative colitis (UC) and Crohn’s disease (CD). Both have chronic and recurrent course, gastrointestinal symptoms, and extraintestinal manifestations. The altered immune response role seems to be important both in UC and CD. In the majority of cases, CD begins with abdominal pain, diarrhea, decrease in appetite, and weight loss; there can be also perianal fistulas, rhagades, and perianal recurrent abscesses. In addition, retarded growth and delayed puberty can precede the development of the disease or can even be predominant at onset. Growth retardation is found in 40% of IBD patients, but the underlying mechanism of this and other extra-intestinal manifestations are partially known: the main hypotheses are represented by malnutrition and inflammatory response during the active phase of the disease. The increased level of pro-inflammatory cytokines can influence growth, but also the onset of puberty and its progression.
Introduction
Inflammatory bowel diseases (IBD) are relevant chronic diseases in children and adolescents characterized by inflammation across the entire gastrointestinal tract and by various clinical presentation and prognosis[1] [1]. In the definition of IBD, three different subtypes are included: ulcerative colitis (UC), Crohn’s disease (CD), and IBD-unclassified[2] [2]. CD affects the entire gastrointestinal tract from the mouth to anus, while UC affects the colon[3] [3]. The incidence of pediatric IBD is also increasing in preschool children, and a rapid increase has been described in some geographic areas (about 7.2% per year)[4] [4]. Pediatric IBD is familial in 19–41% of cases, in fact a positive family history is often present when CD is diagnosed before 11 years of age[3] [3].
The pathogenesis of IBD still needs to be fully clarified, but the role of immune system dysfunction is well known[5][6] [5,6]. In addition, some environmental factors and the modern human lifestyle might be involved in the increasing incidence of IBD[7] [7].
A dysregulated immune response to intestinal bacterial antigens in an individual with a genetic predisposition is one of the explanations for the development of chronic inflammation inside the gut. The intestinal permeability increases because of the breach of the intestinal mucosal barrier, and consequently, there is great exposure of the immune system to antigens, mainly bacteria[8] [8].
In addition, microbiota appears to be altered compared with healthy controls[9] [9]. Microbiota is essential for pathogen protection, nutrition, metabolism, and the immune system, so it is possible that the dysbiosis could be related to IBD pathogenesis, even if a precise relationship has not been fully established yet[10] [10]. In IBD patients, a decrease in bacteria with anti-inflammatory effects and an increase in bacteria that enhance the inflammation have been observed; in particular, it seems that there could be a lower level of Firmicutes and an increase in the presence of Proteobacteria and Bacteroides[11] [11].
The first line of defense against the antigens is the innate immune system; in fact, at first, the wounds of intestinal mucosa are represented by the accumulation in the lamina propria of lymphocytes, plasma cells, Natural Killer (NK) cells, and macrophages by ulcers. Later, the macrophages create ulcers of the entire intestinal mucosa thickness[12] [12,13].
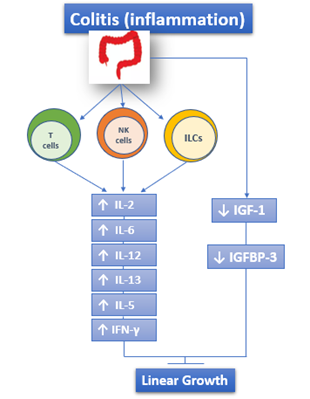
The adaptive immune response is also involved in IBD and is responsible for the chronic inflammatory state. The activity of the adaptive immune system is represented by effector T lymphocytes, regulatory T lymphocytes, and innate lymphoid cells of intestinal mucosa[5][13][14] [5,13,14]. Both UC and CD are characterized by the increased and sometimes massive production of Interleukin (IL)-2, IL-12, IL-6, Interferon γ (IFN-γ), IL-5, and IL-13[15] [15] (Figure 1).
Figure 1. The role of immune response in IBD: both UC and CD are characterized by the increased and sometimes massive production of IL-2, IL-12, IL-6, IFN-γ, IL-5, and IL-13. ILCs: Innate Lymphoid cells.
In addition, microRNAs (miRNA) seem to be involved in the immunopathogenesis of IBD because their dysregulation may result in excessive inflammation[16] [16]. miRNAs are small, single-stranded, noncoding RNA molecules of 18–24 nucleotides that are involved in gene expression modulation at the posttranscriptional level and the current challenge is to identify a precise miRNA that controls a determined gene[17] [17]. Research on IBD and miRNA started in 2008 when Wu et al. identified, for the first time, the miRNA profile in intestinal biopsies from IBD patients[18][19] [18,19].
Since then, the research in this field has been stepped up with a focus on identifying their role as possible biomarkers in IBD diagnosis and as possible target therapy [20]. miRNAs have been shown to regulate specific genes associated with Crohn’s disease (CD) including nucleotide-binding oligomerization domain-containing protein 2 (NOD2), IL-6, and tumor necrosis factor (TNF), but further studies are needed to clarify their precise role and their use in IBD management[20] [20].
Genomic studies have found about 200 loci that could be associated with genetic susceptibility to IBD and all of them encode proteins of the innate and adaptive immune system. In this field, the most important and frequent seems to be a polymorphism in the NOD2 gene[21] [21].
All the cytokines and other mediators of inflammation have a role in the development of intestinal lesions, but they could also be involved in extra-intestinal manifestations of IBD such as cachexia, weight loss, growth failure, and pubertal delay[22] [22].
IBD are systemic disorders and any organ and/or system could potentially be affected by them. Two types of extra-intestinal involvement could be identified: extra-intestinal manifestations (EIM) and extra-intestinal complications. The most frequent EIM are in the joints (peripheral and axial arthropaties), skin (erythema nodosus, pyoderma gangrenosum, Sweet’s syndrome, aphtous stomatitis), hepatobiliary tract (primary sclerosing cholangitis), and eye (episcleritis, uveitis); other EIM could affect rarely the lungs, the heart, the pancreas, and the vascular system[23] [23]. Instead, the complications are caused by all immunogenic mechanisms that characterize the disease and so they are represented by malabsorption, micronutrient deficiencies, osteoporosis, kidney stones, peripheral neuropathies, and gallstones[23] [23].
In general, the course of IBD, and in particular of CD, is more severe in females, but complications like growth failure and puberty delay seem to be more frequent in males[24] [24].
The first-line necessary tests are represented by complete blood count, liver enzymes, albumin, C reactive protein, and/or erythrocyte sedimentation rate[25] [25]. Other useful markers are the fecal calprotectin and lactoferrin, which in any case could be increased in several inflammatory conditions[26] [26]. There are also serological markers like atypical perinuclear antineutrophil cytoplasmic antibody (pANCA) and anti-saccharomyces cerevisiae antibody (ASCA), which are particularly useful in determining the long-term prognosis[27] [27]. Endoscopy is obviously fundamental: the ESPGHAN/NASPGHAN guidelines recommend total colonoscopy, upper endoscopy (esophagogastroduodenoscopy), multiple biopsies, and complete small bowel exploration[28] [28]. Imaging technics such as computer tomography (CT), magnetic resonance enterography (MRE), and small intestine contrast ultrasonography (SICUS) are useful at diagnosis as well as to assess treatment efficacy and disease status[3] [3].
2. Growth Failure and Delayed Puberty in Inflammatory Bowel Diseases (IBD)
Approximately 25% of patients with IBD are diagnosed during childhood and adolescence, and the majority during puberty and pubertal growth spurt[29][30] [29,30].
Symptoms and clinical features of IBD in children and adolescents, at onset and during the disease, are very similar to those in adult patients, but there are two extraintestinal manifestations that characterize IBD in children: growth failure and delayed puberty[31] [31]. Based on studies in the 1980s, 1990s, and 2000s, average standard deviation scores of −0.94 to −1.30 for weight and −0.5 to −1.11 for height at CD onset are reported[32] [32]. The bone development is affected by inflammation and is characterized by low trabecular and high cortical bone density and sometimes by decreased muscle mass. For these reasons, IBD children have high risk of osteopenia and osteoporosis in adulthood[33] [33].
Despite many studies, the underlying mechanisms are not fully understood. The main hypotheses are represented by malnutrition and inflammatory response during the active phase of the disease. The increased level of pro-inflammatory cytokines can impair growth, puberty onset, and its progression[22][34] [22,34].
All the possible risk factors for growth failure and delayed puberty in IBD are shown in Table 1.
Table 1. Possible risk factors for growth failure and delayed puberty in inflammatory bowel diseases (IBD).
|
Probable Risk Factor |
Correlated Factors/Mechanisms of Action |
|
Malnutrition state |
· Malabsorption · Lower caloric intake · Anorexia and poor appetite · Delayed gastric emptying |
|
GH-resistance |
· Circulating IGF-1 decreased levels · Inflammation-induced hepatocyte resistance |
|
Cytokines increased levels |
· Interference with GH signal transduction in the liver · Interference with GH signal transduction in growth plate chondrocytes · Variation of IGF binding protein concentrations · Direct effects on the growth plate |
|
Susceptibility genes |
· Dymeclin gene DYM · Gene OCTN (1/2 variants within IBD5 locus) |
|
Inflammation site (Jejunal disease) |
· Type of disease with less specific symptoms than in colitis one and so the diagnosis might be delayed |
|
Inhibition of the sex steroids production by cytokines |
· Direct action on gonads · Suppression of GnRH secretion |
2.1. Growth Failure
Growth failure can be defined as a static height below the third percentile or a z-score below −2 standard deviations (SD). Another parameter that can be used to evaluate growth problems is height velocity, expressed as a percentile or a SD score according to gender and age [35][36][35,36].
Growth is a marker of health in children and adolescents and growth failure is considered as one of the most important complications of IBD in children[37] [37]: it has been reported in 15–40% of patients with onset of CD in childhood and in 3–10% of patients with onset of UC in childhood, more commonly in males[38][39] [38,39].
In the Sex Differences in Statural Growth Impairment in Pediatric Crohn’s Disease study (also known as the Growth Study), a poorer height growth in males was described: the data suggest that height gain is lower in males than females with skeletal maturation[40] [40].
Children with IBD usually report abdominal pain; they may have malabsorption due to mucosal damage and increased energy requirement because of the inflammatory state. The result is a lower caloric intake and consequently a malnutrition state (with micronutrient deficits—iron, vitamin B12, folate, vitamin D, zinc, other fat-soluble vitamins) that might further impair growth and puberty[41] [41].
Concerning iron deficiency, it is a frequent nutrient deficiency found in adult and pediatric IBD patients and it represents a consequence of malabsorption or intestinal bleeding or dietary restrictions[42] [42]. This type of deficiency can lead to a status of anemia, which is influenced by cytokines (like TNF-α) and by hepcidin. In these situations, hepcidin increases and induces the ferroportin degradation (ferroportin is an enterocyte iron transport protein): in this way, inflammation cytokines may substantially interfere with iron absorption[42] [42]. Vitamin D is very important for normal absorption of calcium and normal bone mineralization[42] [42]. There are insufficient studies on the precise role of vitamin D in IBD in children, but it is probable that it could influence the severity and the course of IBD[43] [43]. Normal bone metabolism could also be affected by vitamin K deficiency, which in some studies on adult patients has been reported with modifications of bone resorption[42][44] [42,44]. Another important micronutrient is zinc: this is absorbed in the small intestine and its deficiency could cause some symptoms including diarrhea and growth failure. Low serum zinc levels have been reported in adolescents with CD compared to controls[42][45] [42,45].
In addition, Ballinger et al.[46] [46] reported anorexia and poor appetite in rats with experimental colitis as a consequence of an increased release of serotonin from the hypothalamus. Furthermore, IL-1 may influence hypothalamic activity and delayed gastric emptying, especially in CD patients[47][48][49] [47–49].
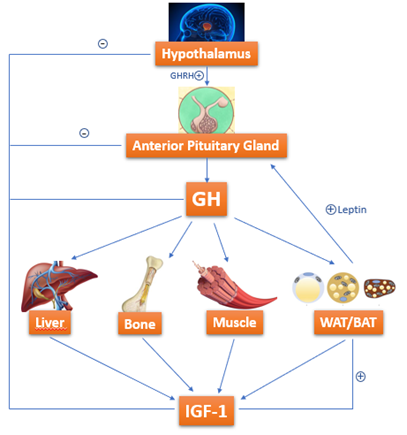
Growth hormone (GH)-resistance is also held liable for growth failure in IBD[50][51] [50,51]. GH is a 191-amino-acid-long polypeptide secreted by somatotropes, which are cells of the anterior pituitary gland[52] [52]. GH is secreted under control of growth hormone releasing hormone (GHRH) and somatostatin, which interact with each other and with several other factors to determine the pulsatile pattern of GH release. In normal conditions, GH stimulates Insulin like Growth Factor-1 (IGF-1) production by the liver. IGF-1 is fundamental in skeletal growth during puberty and bone health during one’s entire life; it increases tissue formation by acting directly and indirectly on target cells and is a crucial mediator of bone growth[52] [52] (Figure 2).
Figure 2. GH-IGF-1 axis: how growth hormone (GH) acts on target organs/tissues, leading to Insulin like Growth Factor-1 (IGF-1) production.
IGF-1 enhances the growth of long bones through the stimulation of proliferation and hypertrophy of chondrocytes of the growth plates [53]. Growth plates in colitis-induced rats are characterized by the inhibition of proliferation and differentiation of cells responsible for growth: these rats seem to have a smaller proliferative zone in their growth plates and reduced terminal hypertrophic chondrocytes zone[54] [54].
Circulating IGF-1 levels are decreased in active CD patients: they depend on several interlacing factors as nutritional status, disease activity, cytokines circulating levels, including tumor necrosis factor (TNF) and IL-1[55] [55]. Among these factors, inflammation seems to have a predominant role: in a recent study, Ballinger et al. analyzed the contribution of undernutrition and inflammation to growth deficit, comparing rats with colitis, healthy free-feeding controls, and a group of animals pair-fed (healthy animals whose daily food intake is matched to the colitis group) to those with colitis. Growth deficit was evident in pair-fed rats compared with healthy free-feeding controls, confirming the important negative effect on growth; in this study, by including a pair-fed group, the authors have tried to separate the effects of undernutrition (occurring in colitic and pair-fed groups in the same way) from inflammation (occurring only in the colitis group) on linear growth. The linear growth was further reduced in the colitis group, suggesting that inflammation may affect by itself growth (or in any case it can enhance the effects of undernutrition). In addition, in these rats, there was a negative correlation between plasma GH and IGF-1 levels, suggesting that there could be an inflammation-induced hepatocyte resistance to GH stimulation[29][56] [29,56]. Gupta et al.[57] [57] showed that IGF-1 levels were lower in males compared to females and that sex differences in growth failure did not depend on diagnosis timing and on timing of pubertal growth spurt.
In addition, children with CD present normal GH secretion with decreased circulating IGF-1 levels, and GH levels were also normal when they have been measured in urine or after stimulation tests: this evidence confirms a possible GH resistance[58][59][60] [58–60].
The role of cytokines can be expressed in four different ways: (1) They might interfere with signal transduction of GH in the liver; (2) there can be a variation of IGF binding protein concentrations; (3) they can have direct effects on growth plate; and (4) cytokines may also interfere with the signal transduction of GH in growth plate chondrocytes[50] [50].
Another hypothesis that is connected with altered IGF-1 function concerns the IGF binding proteins (IGFBPs): indeed, IGF-1 binds to seven different IGFBP and its bioavailability depends on them. The proteins themselves are influenced by the inflammatory status. Among them, the main one is IGFBP-3, and its circulating levels are decreased during active phase of CD and return to normal during the remission phase[61][62][63][64] [61–64] (Figure 1).
Several studies have focused on susceptibility genes for IBD in general and for growth failure specifically[50]. The mainly correlated CD genes seem to be NOD2/CARD15, but they do not influence growth anyway[65].
Several studies have focused on susceptibility genes for IBD in general and for growth failure specifically [50]. The mainly correlated CD genes seem to be NOD2/CARD15, but they do not influence growth anyway [65].
Gene correlation has been described by several authors: Lee et al.[66] reported a significant association between growth failure and a polymorphism in the dymeclin gene DYM, after an analysis of 951 subjects with IBD; Russel et al.[67] showed a possible association of growth impairment with OCTN 1/2 variants within the IBD5 locus. Further studies are needed to clarify this important topic.
Gene correlation has been described by several authors: Lee et al. [66] reported a significant association between growth failure and a polymorphism in the dymeclin gene DYM, after an analysis of 951 subjects with IBD; Russel et al. [67] showed a possible association of growth impairment with OCTN 1/2 variants within the IBD5 locus. Further studies are needed to clarify this important topic.
Another important observation is that the lapse of time between the onset of symptoms and the diagnosis correlates with the severity of growth failure[68][69]. Poor growth (but also BMI and weight), measured by Z scores, persists at follow-up in the very early onset-IBD patients compared with older children who presented growth improvement during follow-up.
Another important observation is that the lapse of time between the onset of symptoms and the diagnosis correlates with the severity of growth failure [68,69]. Poor growth (but also BMI and weight), measured by Z scores, persists at follow-up in the very early onset-IBD patients compared with older children who presented growth improvement during follow-up.
Furthermore, according to another hypothesis, growth retardation could be correlated with the site of the inflammation: patients with jejunal disease have been observed to have more severe growth impairment because they refer less specific symptoms than those with colitis and so the diagnosis might be substantially delayed [32][68].
Furthermore, according to another hypothesis, growth retardation could be correlated with the site of the inflammation: patients with jejunal disease have been observed to have more severe growth impairment because they refer less specific symptoms than those with colitis and so the diagnosis might be substantially delayed [32,68].
Finally, IGF-1 production is also affected by glucocorticoid therapy[70] [71][72].
Finally, IGF-1 production is also affected by glucocorticoid therapy [70–72].
Several studies have investigated the final adult height in patients with childhood-onset IBD: most of them have limitations such as a small number of patients, highly selected study populations, too short study periods, lack of consideration of parental height. The latter limitation is particularly significant because it is well known that parental height is one of the most important determining factors of adult height. A considerable number of IBD subjects will be shorter[73][74]; instead, other data show that the attained adult height is reduced only in a small number of patients with CD, but not in those with UC[75][76].
Several studies have investigated the final adult height in patients with childhood-onset IBD: most of them have limitations such as a small number of patients, highly selected study populations, too short study periods, lack of consideration of parental height. The latter limitation is particularly significant because it is well known that parental height is one of the most important determining factors of adult height. A considerable number of IBD subjects will be shorter [73,74]; instead, other data show that the attained adult height is reduced only in a small number of patients with CD, but not in those with UC [75,76].
In any case, Mouratidou et al.[2] reported that most patients with childhood onset IBD had a low attained adult height, but the final height only seemed to be modestly lower than that of healthy peers and siblings. Therefore, high levels of severe inflammation markers are associated with reduced final adult height, so, perhaps, in these cases, patients have to be treated earlier or in a more aggressive way.
In any case, Mouratidou et al. [2] reported that most patients with childhood onset IBD had a low attained adult height, but the final height only seemed to be modestly lower than that of healthy peers and siblings. Therefore, high levels of severe inflammation markers are associated with reduced final adult height, so, perhaps, in these cases, patients have to be treated earlier or in a more aggressive way.