Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Diana Pinto.
The seaweeds or macroalgae belong to the basic tropic level in the marine water ecosystem and are responsible, with microalgae, for the balance of the abiotic and biotic factors of marine life. Seaweeds represent a valuable resource of bioactive compounds associated with anti-inflammatory effects and offer great potential for the development of new anti-inflammatory drugs.
- seaweed
- specialized metabolites
- phlorotannins
- bromophenols
- chromenes
- terpenoids
- fucoxanthin
- fucosterol
- caulerpin
- fatty acids
1. Introduction
Macroalgae use is increasing and spreading. What was a common food ingredient in Oriental cuisine is nowadays an additive in several smart foods and folk medicine formulations. Some formulations are sold as promoters of health benefits, including anti-inflammatory effects. Although several macroalgae extracts, as stated above, showed anti-inflammatory activity, their specialized metabolites must also be tested, and their amount in the formulations must be established. Moreover, in vivo studies and clinical trials are still required to validate the claimed potential in pharmaceutical formulations. Knowing the limitations of the in vitro studies, several authors are moving forward and focusing their biological assays using in vivo models. Unfortunately, anti-inflammatory clinical trials are still needed. This text aims to give a critical synopsis of the current state of the art regarding the anti-inflammatory effects of essential macroalgae specialized metabolites, emphasizing their molecular mechanisms. The following sections will present and discuss specific examples and consider the most promising anti-inflammatory compounds, as well as the ones for which the studies are broadening, including mechanism of action, and, if possible, in vivo studies.
2. Phlorotannins
Phlorotannins are polymers of benzene-1,3,5-triol (1), commonly named phloroglucinol (Figure 1). The polymerization occurs through a single ether bond, 1,4-dibenzodioxin linkage, or by direct covalent bond between the benzene rings [11,33][1][2]. Phlorotannins have a wider mass range (from 125 to 1 × 105 Da or higher) and different structures. They can be divided into four classes: fuhalols and phlorethols (ether bond), fucols (phenyl bond), fucophloroethols (ether and phenyl bonds), and eckols and carmalols (a dibenzodioxin bond). They are found in high quantities in brown algae and are considered the specialized metabolites responsible for the pharmacological activities of some Ecklonia and Eisenia species, such as Ecklonia cava, Kjellman, 1885 [34,35][3][4]. Phlorotannins have demonstrated their anti-inflammatory effects by inhibiting hyaluronidase, phospholipase A2, lipoxygenase, and cyclooxygenase (COX) enzymes, which are involved in the inflammatory response, as well as chemical mediators and pro-inflammatory cytokines [36,37,38,39][5][6][7][8].
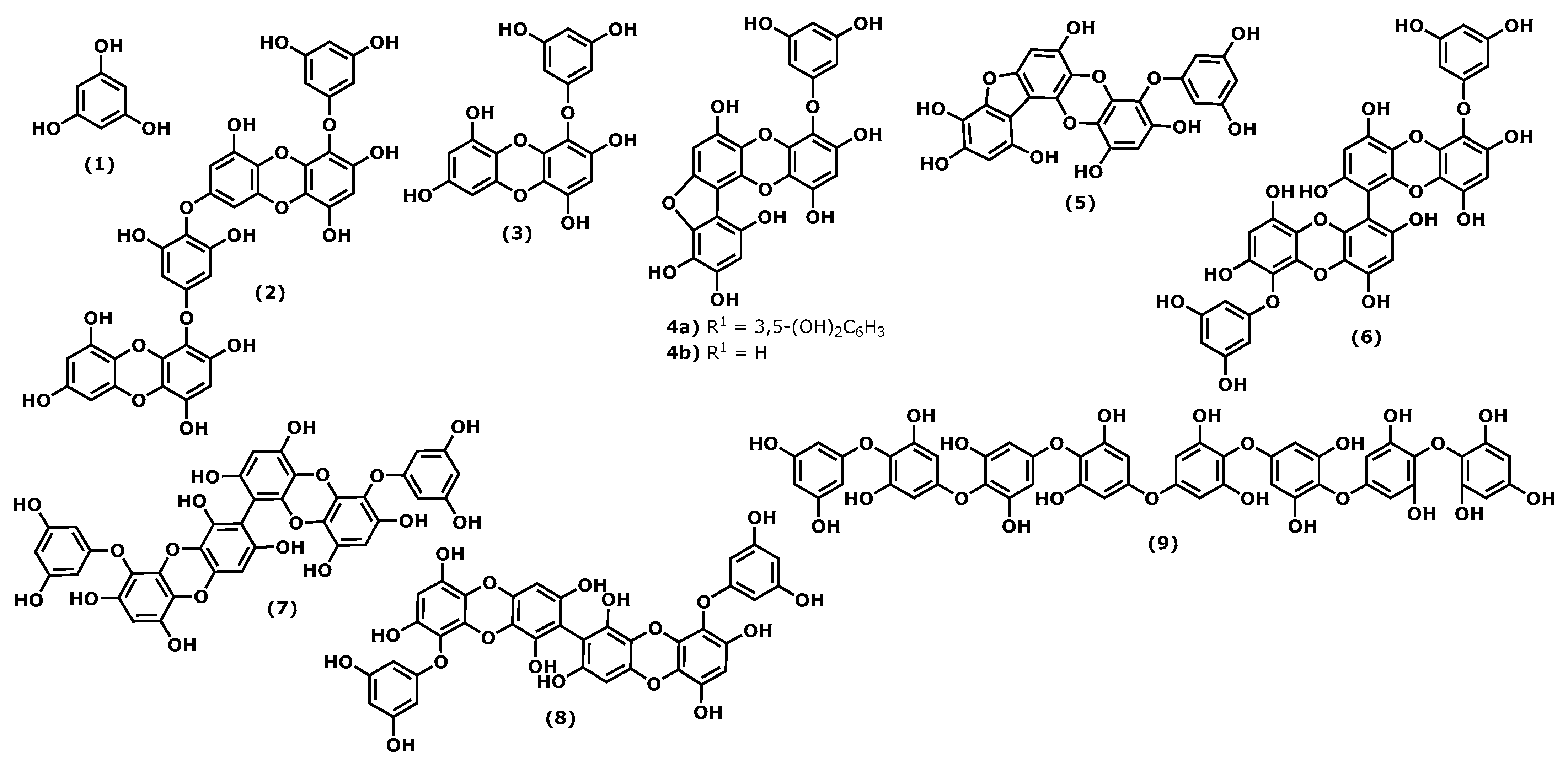
Figure 1.
Structures of the phlorotannins whose anti-inflammatory activity is discussed.
Considering the phloroglucinol (1) (Figure 1) anti-inflammatory effects, it is worth mentioning that inhibitory effects on oxidative stress were reported, and phloroglucinol (1) is also able to inhibit the production of tumour necrosis factor-α (TNF)-α, interleukin-1β, interleukin-6 ((IL)-1β), and IL-6, and prostaglandin E(2) (PGE2) in lipopolysaccharide (LPS)-stimulated RAW264.7 cells. Additionally, phloroglucinol decreased the expression of matrix metalloproteinases (MMPs) in the human fibrosarcoma cell line HT1080. MMPs are known to be involved in several inflammatory conditions. Finally, phloroglucinol inactivates the NF-κB-inducing kinase (NIK) and the kinases ERK and MAPK, preventing inflammation episodes [40][9].
Dieckol (4-(4-((6-(3,5-dihydroxyphenoxy)-4,7,9-trihydroxydibenzo[b,e][1,4]dioxin-2-yl)oxy)-3,5-dihydroxyphenoxy)dibenzo[b,e][1,4]dioxine-1,3,6,8-tetraol (2)), phlorofucofuroeckol(PFF) B (4-(3,5-dihydroxy-phenoxy)benzo[b]benzo[5,6][1,4]dioxino[2,3-e]benzofuran-1,3,6,9,10,12-hexaol (4b)), and fucofuroeckol-A (4-(3,5-dihydroxyphenoxy)benzo[b]benzo[5,6][1,4]dioxino[2,3-e]benzofuran-1,3,6,9,10,12-hexaol (5)) (Figure 1) suppresses lipopolysaccharide (LPS)-induced production of NO, PGE2 and expression of pro-inflammatory proteins (nitric oxide synthase (iNOS), COX-2, tumour necrosis factor (TNF)-α and interleukin (IL)-1β, and IL-6) in a dose-dependent manner in RAW 264.7 macrophages and BV2 microglia cells. Normally, the inhibition profile of these compounds is associated with their ability to inhibit NF-κB and p38 mitogen-activated protein kinases (MAPKs) activation [30,37,39,41][6][8][10][11].
In a comparative study of the effects of dieckol (2) and eckol (4-(3,5-dihydroxyphenoxy)dibenzo[b,e][1,4]dioxine-1,3,6,8-tetraol (3)) (Figure 1) on LPS-mediated hyperpermeability and monocytes migration in human umbilical vein endothelial cells (HUVECs), it was observed that dieckol (2) has a better inhibitory effect than eckol (3), probably due to the higher number of hydroxy groups in the dimeric structure [42][12]. However, Eom et al. [43][13] showed that eckol (3) could inhibit the expression of various inflammatory cytokines in Propionibacterium acnes-induced human skin keratinocytes (HaCaT) cells. It inhibited the expression levels of MMPs (MMP-2 and -9), inflammatory mediators in a concentration-dependent manner, acting at the transcriptional level. Its anti-inflammatory properties were associated with the inhibition of p-NF-κB p65 and p-Akt at the translational level [43][13].
In the last decade, it was demonstrated that 6,6′-bieckol (6,6′-bis(3,5-dihydroxyphenoxy)-[1,1′-bidibenzo[b,e][1,4]dioxin]-2,2′,4,4′,7,7′,9,9′-octaol (6)) (Figure 1), isolated from E. cava, inhibited the expression and release of NO, PGE2, TNF-α, and IL-6 in LPS-stimulated macrophages, with concomitant inhibition of NF-κB activation [44][14]. More recently, Sugiura et al. [45][15] demonstrated using a rat mast cell line (RBL-2H3) that 6,6′-bieckol (6) and other phlorotannins such as eckol (3), 6,8′-bieckol (6,9′-bis(3,5-dihydroxyphenoxy)-[1,2′-bidibenzo[b,e][1,4]dioxin]-1′,2,3′,4,6′,7,8′,9-octaol (7)), 8,8′-bieckol (9,9′-bis(3,5-dihydroxyphenoxy)-[2,2′-bidibenzo[b,e][1,4]dioxin]-1,1′,3,3′,6,6′,8,8′-octaol (8)), PFF-A (4,9-bis(3,5-dihydroxyphenoxy)benzo[b]benzo[5,6][1,4]dioxino[2,3-e]benzofuran-1,3,6,10,12-pentaol (4a)), and PFF-B (4b) isolated from Eisenia arborea, Areschoug, 1876, suppressed the release of chemical mediators (histamine, leukotriene B4, and PGE2) COX-2 mRNA expression and inhibited COX-2 activity at a 500 μM of concentration [45][15].
Octaphlorethol A (2-(4-(4-(4-(4-(4-(4-(3,5-dihydroxyphenoxy)-3,5-dihydroxyphenoxy)-3,5-dihydroxyphenoxy)-3,5-dihydroxyphenoxy)-2,6-dihydroxyphenoxy)-2,6-dihydroxyphenoxy)-2,6-dihydroxyphenoxy)benzene-1,3,5-triol (9)) can be isolated from the brown marine alga Ishige foliacea, Okamura, 1936, and presents anti-inflammatory potential by inhibiting the CpG-stimulated primary murine bone marrow-derived macrophages and dendritic cells. The pre-treatment with octaphlorethol A (9) caused strong inhibition of IL-12 p40, IL-6 and TNF-α production, indicating the inhibitory effect of this compound on pro-inflammatory cytokine production. It also demonstrated the inhibitory effect on TLR9-dependent MAPK and NF-ƘB activation [46][16].
In terms of in vivo assays, they can be considered scarce, and more studies are needed; moreover, several aspects of the phlorotannins’ mechanisms of action need to be clarified. The leukocyte adhesion of endothelial cells and transendothelial migration (TEM) of leukocytes are essential steps in the pro-inflammatory response. In in vivo assays, both compounds (2) and (3) exhibited an effectively inhibitory effect on the leakage of dye into the peritoneum in mice and decreased leukocytes count at a dose of 10 μΜ of concentration [47][17].
The phlorotannins eckol (3), 6,6′-bieckol (5), 6,8′-bieckol (6), 8,8′-bieckol (7), PFF-A (4a), and PFF-B (4b) were administrated orally to mice, which were previously injured using arachidonic acid (AA), 12-O-tetradecanoylphorbol-13-acetate (TPA) and oxazolone (OXA), and proved to be able to suppress the AA, TPA, and OXA-induced mouse ear swelling. Moreover, their positive effect was better than the epigallocatechin gallate (EGCG), which was used as the positive control influence. The 6,8′-bieckol at 75 nmol exhibited the most potent suppression (77.8%) compared with the epigallocatechin gallate’s weakest suppression (5.7%) [44][14].
Phlorotannins are one of the most studied macroalgae-derived metabolites; nevertheless, their potential use as new anti-inflammatory drugs needs additional studies, such as pharmacokinetic and clinical trials.
3. Bromophenols
Bromophenols are phenols bearing bromine and hydroxy groups in one or more benzene rings and are amongst the specialized metabolites produced by macroalgae [48][18]. Actually, bromophenols are ubiquitous in the three types of macroalgae [49[19][20],50], although they were first found in red Rhodomela larix (Turner), C. Agardh, 1822, (the current accepted name is Neorhodomela larix (Turner), Masuda, 1982) [51][21]. Several pharmaceutical potentials have been reported for these natural compounds [50][20]; however, their anti-inflammatory properties were scarcely explored.
Vidalol A and B (2-bromo-4-(2,3-dibromo-4,5-dihydroxybenzyl)benzene-1,3,5-triol (10) and 2-bromo-4,6-bis(2,3-dibromo-4,5-dihydroxybenzyl)benzene-1,3,5-triol (11)) (Figure 2), isolated from the red algae Vidalia obtusiloba (Mertens ex C. Agardh), J. Agardh, 1863, are examples of compounds that inhibit the bee venom-derived phospholipase A2 (PLA2), showing 96% enzyme inactivation at 1.6 μg/mL. An in vivo assay in the phorbol ester (PMA)-induced swelling mouse ear showed that (10) and (11) reduced oedema (58–82%) significantly when applied topically [52][22].

Figure 2.
Structures of the bromophenols whose anti-inflammatory activity is discussed.
The 3-bromo-4,5-dihydroxybenzaldehyde (3-BDB) (12) (Figure 2) is another example of a bromophenol (isolated from marine red algae, such as Polysiphonia morrowii, Harvey, 1857, Polysiphonia urceolata (Lightfoot ex Dillwyn), Greville, 1824, and Rhodomela confervoides (Hudson), P. C. Silva, 1952 [53,54][23][24]) that in LPS-stimulated RAW 264.7 murine macrophages can suppress the production of IL-6, a pro-inflammatory cytokine, in a dose-dependent manner. BDB also had an inhibitory effect on the phosphorylation of nuclear factor NF-κB, a signal transducer and activator of transcription 1 (STAT1; Tyr 701), which are two major signalling molecules involved in cellular inflammation. The in vivo assay of BDB (12) on atopic dermatitis (AD) in BALB/c mice induced by 2,4-dinitrochlorobenzene (DNCB) showed that treatment (100 mg/kg) resulted in suppression of the development of AD symptoms compared with the control treatment. 3-BDB (12) also reduced immunoglobulin E levels in serum, smaller lymph nodes with reduced thickness and length, decreased ear oedema, and reduced levels of inflammatory cell infiltration in the ears [55][25]. With a similar structure to BDB (12), 3-bromo-5-(ethoxymethyl)benzene-1,2-diol (BEMB) (13) (Figure 2), also isolated from the red algae P. morrowii. BEMB (13) demonstrated anti-inflammatory effects by inhibiting the production of NO, the expression of iNOS, and COX-2 in the LPS-activated RAW 264.7 cells and zebrafish embryos without cytotoxicity. It suppressed the protein and mRNA expression levels of nuclear factor NF-ƘB in the LPS-activated RAW 264.7 cells and zebrafish model [56][26]. The last example is bromophenol bis(3-bromo-4,5-dihydroxybenzyl) ether (BBDE) (14) (Figure 2) isolated from the same algae, which can inhibit inflammation by reducing inflammatory mediators, such as NO, prostaglandin E2, iNOS, COX-2, and pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6), in LPS-induced RAW 264.7 macrophage cells [57][27].
Considering the number of bromophenols found in macroalgae, their anti-inflammatory potential is scarcely studied; more toxicological and in vivo studies are needed, and, in some cases, clinical trials would be appreciated.
4. Chromenes
Chromenes or benzopyrans represent the basic nucleus of various seaweed compounds with an anti-inflammatory potential, which includes the inhibition of COX and lipoxygenase, enzymes linked to inflammatory manifestations.
The 2-acetoxy-2-(5-acetoxy-4-methyl-2-oxotetrahydro-2H-pyran-4-yl)ethyl 4-(3-methoxy-2-methoxymethyl-7-ethyl-3,4,4a,7,8,8a-hexahydro-2H-chromen-4-yloxy)-5-methyl-heptanoate (15) (Figure 3), isolated from the red seaweed Gracilaria opuntia, Durairatnam, nom. Inval., 1962, showed a moderate anti-inflammatory activity against the COX-2 isoform (IC50 0.96 mg/mL) than COX-1 (IC50 1.21 mg/mL), in comparison to the traditional NSAID, such as aspirin (anti-COX-1 IC50 0.005, anti-COX-2 IC50 0.21 mg/mL) and ibuprofen (anti-COX-1 IC50 0.04 mg/mL, anti-COX-2 IC50 0.09 mg/mL). The in vitro 5-lipoxygenase (5-LOX) activity (IC50 1.22 mg/mL) was comparable to that of synthetic ibuprofen (IC50 0.93 mg/mL) [58][28]. Further, isolated from G. opuntia, the 5-[7-(5-ethyl-3,4-dimethoxycyclooctyl)benzofuran-6-yl]-7-methyl-3,4,7,8-tetrahydro-2H-oxocin-2-one (16) and 2-(3-ethyl-9-(2-methoxyethoxy)-1-oxo-2,3,4,9-tetrahydro1H-xanthen-2-yl)ethyl 5-hydroxy-9-methoxy-7,8-dimethyl-8-(5-methylfuran-2-yl)nona-3,6-dienoate (17) (Figure 3) exhibited inhibitory activities towards pro-inflammatory cyclooxygenase-2/5-lipoxygenase (COX-1, 2, and 5-LOX). Both compounds had a comparable inhibitory effect in 5 LOX (IC50 0.209 × 10−2 M) with synthetic non-steroidal anti-inflammatory drugs (NSAID) ibuprofen (IC50 0.451 × 10−2 M, p < 0.05) and selectivity towards COX inhibition (SI: anti-COX-1 IC50/anti-COX-2 IC50 ~1.08–1.09) than NSAID (aspirin, and ibuprofen, SI: 0.02 and 0.44, respectively, p < 0.05) [59][29].

Figure 3.
Structures of the chromene derivatives whose anti-inflammatory activity is discussed.
4′-[10′-[7-Hydroxy-2,8-dimethyl-6-(pentyloxy)-2H-chromen-2-yl]ethyl]-3′,4′-dimethyl-cyclohexanone (18) and 3′-[10′-(8-hydroxy-5-methoxy-2,6,7-trimethyl-2H-chromen2-yl)ethyl]-3′-methyl-2′-methylene cyclohexyl butyrate (19) (Figure 3), isolated from the red seaweed Gracilaria Salicornia (C. Agardh), E. Y. Dawson, 1954, were tested against pro-inflammatory 5-LOX, and compound (19) registered significantly higher activity (IC50 2.03 mM) than that displayed by (18) (IC50 2.46 mM, p < 0.05). The compound selectivity index was also higher (IC50 anti-COX-1/IC50 anti-COX-2 > 0.95) than that exhibited by the non-steroidal anti-inflammatory agent ibuprofen (0.89) (p < 0.05). These studies suggested that chromenyls have higher selectivity towards inducible pro-inflammatory COX-2 than its constitutive isoform COX-1 [60][30].
Concerning phenolic compound, rutin (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-(((2S,4S,5S,6R)-3,4,5-trihydroxy-6-((((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)methyl)tetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one (20)) (Figure 3), identified in the crude extract of Porphyra dentata, Kjellman, 1897, (the current accepted name Neoporphyra dentata (Kjellman), L.-E. Yang and J. Brodie) inhibited NO production in LPS-stimulated RAW 264.7 cells. Its activity was compared to the obtained for catechol, and it was observed that catechol was a more potent suppressor of the up-regulation of iNOS promoter and NF-κB enhancer than rutin (20). Catechol (1–11 μg/mL) inhibited iNOS promoter activity to a greater extent than rutin (80–250 μg/mL) in a dose-dependent manner. Catechol (11 μg/mL) and rutin (250 μg/mL) decreased LPS-induced NF-κB enhancer activity to six- and twofold, respectively [61][31]. It is relevant that some of these metabolites were evaluated for their selectivity index; however, few action mechanisms were revealed, and in vivo studies are suggested.
5. Terpenoids
Terpenoid is a general term for hydrocarbons and their oxygen-containing derivatives obtained through isoprene unit polymerization. They are usually classified into monoterpenes, sesquiterpenes, diterpenes, and polyterpenes according to their structural units [62][32] and are recognized for their biological activities, from which anticancer can be highlighted [63][33].
5β-Hydroxypalisadin B [(2R,5R,7S,9aS)-7-bromo-2-(bromomethyl)-3,6,6,9a-tetramethyl-2,5,5a,6,7,8,9,9a-octahydrobenzo[b]oxepin-5-ol (21)] (Figure 4), a sesquiterpene isolated from the red algae Laurencia snackeyi (Weber Bosse), M. Masuda, 1997, [64][34], suppressed the NO production, iNOS, and COX-2 expression and cytokine release in LPS-stimulated RAW 264.7 cells. Moreover, LPS-induced NO production was dose-dependently decreased with a maximum of 90% inhibition observed at the concentration of 50 µM [64][34]. The in vivo studies performed in lipopolysaccharide (LPS)-induced zebrafish embryo using 0.25, 0.5, and 1 μg/mL of compound (21) showed a profound protective effect of this compound in the zebrafish embryo as confirmed by survival and heartbeat rate, and yolk sac oedema size. It inhibited the LPS-induced NO production in a dose-dependent manner. Moreover, 5β-hydroxypalisadin B (21) showed a protective effect compared to dexamethasone, the standard anti-inflammatory agent [65][35].
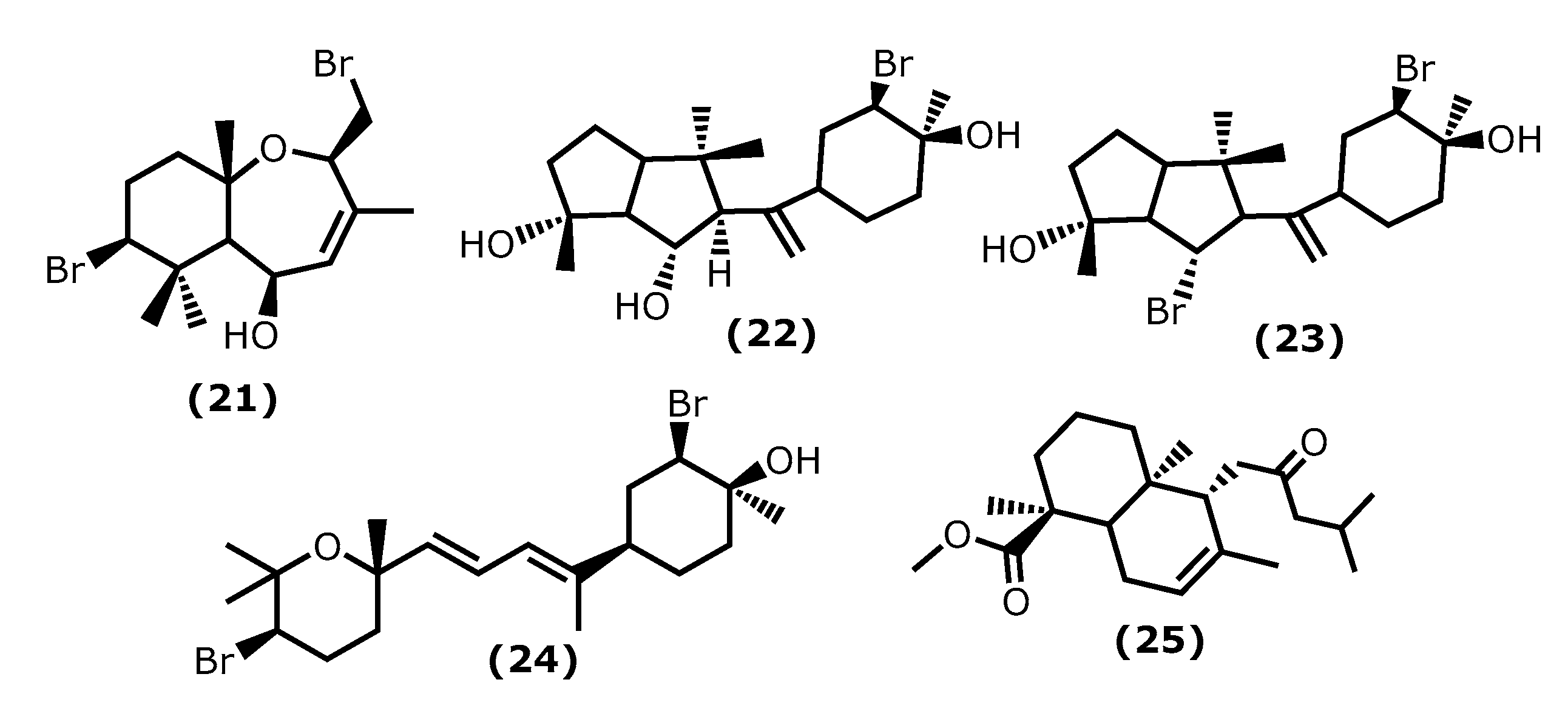
Figure 4.
Structures of the terpenoids whose anti-inflammatory activity is discussed.
Neorogioltriol ((1R,5S,6S)-5-(1-((3R,4S)-3-bromo-4-hydroxy-4-methylcyclohexyl)vinyl)-1,4,4-trimethyloctahydropentalene-1,6-diol (22)) (Figure 4) is a tricyclic diterpenoid isolated from the red algae Laurencia glandulifera (Kützing), Kützing, 1849, ref. [66][36] and was evaluated using the writhing test, showing that 1 mg/kg (b.w.) was enough to reduce the mouse acetic acid-induced writhing response by 88.9% [66][36]. The in vivo tests using formalin-induced licking in rats showed that compound (22) affected neurogenic and/or inflammatory pain. Neorogiotriol (22) exhibited a remarkable reduction in the licking time by 48% in the second phase, which begins at 20 min and can last up to 60 min, representing inflammatory pain. This inhibition effect obtained in the second phase of the performed test is typical of COX inhibitors, suggesting peripheral analgesic activity [66][36].
Neorogioldiol ((1R,6S)-6-bromo-5-(1-((3R,4S)-3-bromo-4-hydroxy-4-methylcyclohexyl)vinyl)-1,4,4-trimethyloctahydropentalen-1-ol (23)) and O11,15-cyclo-14-bromo-14,15-dihydrorogiol-3,11-diol (24) (Figure 4) are two brominated diterpenoids found in Laurencia sp., and, together with compound (22), were assessed for their anti-inflammatory capacity in vitro using RAW 264.7 cells [67][37]. Compounds (23) and (24) were also evaluated in vivo using C57BL/6J mice with dextran sodium sulphate (DSS)-induced inflammatory bowel disease (colitis) [67][37]. All compounds (22–24) suppress macrophage activation and promote an M2-like anti-inflammatory phenotype by inducing expression of arginase 1, MRC1, IRAK-M, the transcription factor C/EBPβ, and the miRNA miR-146a; also, they suppressed iNOS induction and NO production [67][37]. The C57BL/6J mice received 2.5% DSS in their drinking water and were injected intraperitoneally with compounds (23) and (24) every second day for 5 days. All DSS-treated mice showed a reduction in colon length, which confirms colonic inflammation macroscopically, as well as a very significant decrease in pro-inflammatory cytokine messenger RNA (mRNA) (more than a 40-fold decrease in the case of interleukin-6) [67][37].
Lastly, the diterpenoid methyl 16(13→14)-abeo-7-labdebe(12-oxo)carboxylate (25) (Figure 4) isolated from the red algae G. salicornia presented a similar anti-inflammatory effect against pro-inflammatory 5-LOX (IC50 0.86 mg/mL) comparative to the ibuprofen (IC50 0.92 mg/mL, p < 0.005) [68][38].
6. Fucoxanthin
Fucoxanthin ((3R)-3-hydroxy-4-((3E,5E,7E,9E,11E,13E,15E)-18-((1S,4S,6R)-4-hydroxy-2,2,6-trimethyl-7-oxabicyclo[4.1.0]heptan-1-yl)-3,7,12,16-tetramethyl-17-oxooctadeca-1,3,5,7,9,11,13,15-octaen-1-ylidene)-3,5,5-trimethylcyclohexyl acetate (26)) (Figure 5) is the most abundant natural carotenoid, accounting for approximately 10% of nature’s carotenoids. It is found mainly in brown algae and structurally contains allene bonds, 5,6-monocyclic oxide, and acetylated groups. Beneficial health effects have been reported for fucoxanthin (26), the reason why it is one of the most studied metabolites [69][39]. Regarding the anti-inflammatory effect, the literature survey indicates that fucoxanthin has a protective effect on various inflammation-related diseases. From which diabetes [70[40][41],71], neurodegenerative [72[42][43][44],73,74], skin and liver [75[45][46],76], inflammatory pain [77][47], and cardiovascular [78,79][48][49] can be highlighted.

Figure 5.
Fucoxanthin structure.
Recent studies showed that fucoxanthin has a significant pharmacological effect on diseases related to oxidative stress injury. Its mechanism of action is primarily related to nuclear factor-erythroid 2-related (Nrf2) signal transduction pathway and gut microbiota regulation [80][50]. Zheng et al. [81][51] showed that fucoxanthin increased the phosphorylation level of the Akt/Nrf2 pathway as well as its effect on increased the mRNA and proteins levels of glutamate-cysteine ligase catalytic subunit (GCLC) and glutathione synthetase (GSS) in human keratinocytes (HaCaT) [81][51].
Su et al. [82][52] demonstrated that fucoxanthin has a tremendous anti-inflammatory effect in a mouse sepsis model. LPS was used to induce sepsis in mice; when treated with 1 mg/kg (b.w.) of fucoxanthin, the survival rate can duplicate (20% to 40%). Fucoxanthin is related to the reduced levels of the pro-inflammatory cytokines’ TNF-α and IL-6 and the inhibition of the NF-ƘB inflammatory pathway [82][52].
Knowing the anti-inflammatory properties of fucoxanthin, Wu et al. [83][53] produced a nanofiber membrane named PLA/PEGDA-EDT@rGO-fucoxanthin (PPGF) that can capture ROS. Poly(ethyleneglycol)diacrylate(PEGDA)-1,2-ethanedithiol (EDT) copolymer (PEGDA-EDT) is responsible for the ROS capture, reduced graphene oxide (rGO) is the drug carrier, and fucoxanthin (26) attenuates osteoarthritis (OA) [83][53]. In response to hydrogen peroxide, the nanofiber membrane exhibited sustained and long-term fucoxanthin release behaviour in vitro (at least 66 days). Moreover, it showed low cytotoxicity and exceptional ability to capture ROS. PPGF showed excellent anti-inflammatory and antioxidant effects on IL-1β-induced chondrocytes by potent ROS scavenging; however, it is possible that its mechanism of action also involves the upregulation of antioxidative enzymes [83][53].
7. Fucosterol
Fucosterol ((3S,10R,13R,17R)-17-((2R)-5-hydroxy-5-isopropylhept-6-en-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol (27)) (Figure 6) is one of the dominant sterols in marine macroalgae. Brown macroalgae contain higher levels of fucosterol (27) than green and red macroalgae. It can be found in brown macroalgae and isolated from species of the genera Laminaria, Undaria, Sargassum, and Ecklonia [84,85][54][55]. It is known to present several health benefits [86][56], and it is also known that fucosterol (27) has effects on several inflammatory pathways, such as decreasing the expression of p50 and p65 mRNA and the activity of NF-κB promoter in a dose-dependent manner, inhibiting the expression of TNF-α, COX-2, IL-1β, and IL-6 [87,88][57][58]. It also reduced the inflammatory response caused by solar ultraviolet radiation (UVR) [89][59].

Figure 6.
Fucosterol structure.
More specific assays showed that fucosterol (27) protects LPS-induced acute lung injury (ALI) in mice [90][60]. The mechanism of action was revealed to be through the inhibition of TNF-α, IL-1β, and IL-6 levels in the bronchoalveolar lavage fluid (BALF) and the LPS-stimulated alveolar macrophages, reducing their expression by about 50%, when compared to the untreated group [90][60].
Sun et al. [91][61] demonstrated the protective mechanisms of fucosterol (27) on cobalt chloride (CoCl2)-induced hypoxia damage to keratinocytes (HaCaT). It attenuates CoCl2-induced excess expression of IL-6, IL-1β, and TNF-α and suppresses the phosphorylation of PI3K and Akt and the accumulation of HIF1-α simulated by CoCl2 [91][61]. On the other hand, Mo et al. [92][62] showed that (27) attenuated serum liver enzyme levels, hepatic necrosis, and apoptosis induced by TNF-α, IL-6, and IL-1β. It also showed the effect of this compound in the reduction in P38 MAPK, and NF-κB signalling was accompanied by PPARγ activation [92][62].
In the last years, Wong et al. [93][63] showed that (27) protects against amyloid β (Aβ)-mediated neuroinflammation by inhibiting the production of IL-6, IL-1β, TNF-α, NO, and PGE2 in LPS- or Aβ-induced microglial cells. Moreover, a similar study reported the fucosterol (27) effect on attenuate particulate matter CPM-induced inflammatory responses in A459 human lung epithelial cells through lowering the P65 and P50 nuclear translocation and the p38 mitogen-activated protein kinase (MAPK) phosphorylation, extracellular signal-regulated kinases 1/2 (ERK1/2) and c-Jun N-terminal kinases (JNK), and the levels of COX-2, PGE2, TNF-α, and IL-6 [94][64].
8. Caulerpin
Caulerpin (dimethyl(6E,13E)-5,12-dihydrocycloocta [1,2-b:5,6-b′]diindole-6,13-dicarboxylate (28)) is an alkaloid found in seaweeds and presents desirable anti-inflammatory activity mainly attributed to indole moiety. The two indole units are linked together by a cyclooctane ring forming the 5,12-dihydrocycloocta [1,2-b:5,6-b′]diindole nucleus with two methoxycarbonyl groups at C-6 and C-13 (Figure 7) [95,96][65][66].
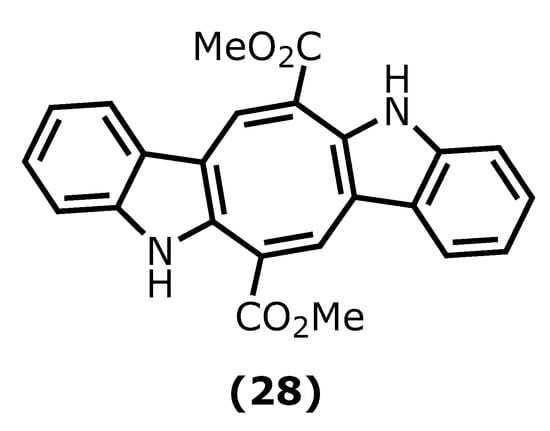
Figure 7.
Caulerpin structure.
Caulerpin (28) has been isolated mainly from green and red algae species, such as Caulerpa racemose (Forsskål), J. Agardh, 1873, Caulerpa sertularioide (S. G. Gmelin), M. Howe, 1905, and Caulerpa mexicana, Sonder ex Kützing, 1849, and its in vivo anti-inflammatory activity has been investigated. For instance, its potency against ear oedema and peritonitis in mice induced by capsaicin (8-methyl-N-vanillyl-6-nonenamide) and carrageenan was assessed. Caulerpin (28) caused a significant reduction in plasma extravasation of mice ears (55.8%), when compared to capsaicin and leukocyte reduction [97][67].
Lucenna et al. [98][68] also reported the caulerpin (28) anti-inflammatory effect on the murine model of peritonitis and ulcerative colitis. The authors established that caulerpin (28) at 4 mg/kg triggered improvement of the Disease Activity Index (DAI) and attenuated the colon shortening and damage. This dose reduced the TNF-α, IFN-γ, IL-6, IL-17, and NFκB p65 levels and increased the levels of IL-10 in the colon tissue [98][68].
9. Fatty Acids
Fatty acids (FAs) are classified according to their carbon-chain length and sometimes the number of double bonds present. Long-chain fatty acids should have more than twelve carbons in the chain, whereas very long-chain should contain more than twenty-two. In the case of the polyunsaturated fatty acids, a further classification of omega-3 (ω-3) and omega-6 (ω-6), based on the position of the first double bond on the methyl terminal end, can be found in the literature. Polyunsaturated fatty acids (PUFAs) are known to play a vital role in body homeostasis. In general, higher levels of ω-6 polyunsaturated fatty acids are associated with constriction of blood vessels, inflammation, and platelet aggregation, whereas ω-3 may help to resolve inflammation and alter the function of vascular biomarkers [99][69]. It is known that ω-3 PUFAS has an important role in the reduction of depressive symptoms and exerts an anti-inflammatory action by the production of distinct metabolites, such as resolvins D (RvD) and E series, and maresins (MaR) and protectins (PD). The Z-4,7,10,13,16,19-docosahexaenoic acid (DHA)-derived trihydroxydocosahexanoic acid mediators termed RvD are produced by a series of reactions involving COX-2 and 5-LOX or by a pathway involving lipoxygenase enzymes and other reactions. The metabolism of DHA initially occurs by 15-lipoxygenase and then a series of other reactions generates a dihydroxy derivative termed protectin D1. The trihydroxyeicosapentaenoic acid mediators, termed RvE, form from Z-5,8,11,14,17- eicosapentaenoic acid (EPA) by a similar series of reactions involving COX-2 and 5-LOX [100][70]. These mediators appear to act as a potent anti-inflammatory in psychiatric, neurodegenerative, and neurological diseases. On a cellular level and in a depression model, RvDs increased serotonin levels; on the other hand, they decreased gliosis in neurodegenerative disorders. Protectins prevented neurite and dendrite retraction and apoptosis in models of neurodegeneration, whereas maresins reduced cell death [101][71].
Palmitic acid, a saturated fatty acid, is the most prevalent in seaweeds; nevertheless, ω-6 and ω-3 PUFAs [99][69], such as DHA (29), EPA (30), Z-6,9,12,15-octadecatetraenoic acid (stearidonic acid-SA) (31), Z-8,11,14,17-eicosatrienoic acid (ETA) (32), and Z-5,8,11,14-icosa-5,8,11,14-tetraenoic acid (arachidonic acid-AA) (33) (Figure 8), are also commonly isolated from seaweeds [99,102][69][72]. There is evidence that these molecules can play a key role in the inflammation process [100,103][70][73]. Pro-inflammatory PGE2 and leukotriene B4 (LTB4) are produced during the metabolism of AA (33) through COX and 5-lipoxygenase of leukotriene-A4 (LTA4) hydrolase enzymatic pathway, respectively. At the same time, DHA (29) and EPA (30) compete with AA (33) metabolism, thus reducing the production of PGE2 and LTB4. The metabolism of ETA (32) by 5-lipoxygenase form leukotriene-A3 (LTA3) inhibits the LTA4 hydrolase necessary for the production of LTB4, thus acting as an anti-inflammatory by inhibiting LTB4 production [104,105][74][75]. Moreover, SA (31) and EPA (30), which were extracted from Undaria pinnatifida (Harvey), Suringar, 1873, exhibited anti-inflammatory activity against mouse ear enema, erythema, and blood flow induced by phorbol myristate acetate. Whereas SA (31) extracted from Ulva pertusa, Kjellman, 1897, was able to suppress the production of LTB4, Leukotriene C4 (LTC4), and 5-hydroxyeicosatetraenoic acid (5-HETE) in an MC/9 mouse mast cell [99,100,101,102,103,104,105][69][70][71][72][73][74][75].

Figure 8.
Fatty acids structures.
References
- Freitas, M.V.; Pacheco, D.; Cotas, J.; Mouga, T.; Afonso, C.; Pereira, L. Red seaweed pigments from a biotechnological perspective. Phycology 2022, 2, 1–29.
- Okada, Y.; Ishimaru, A.; Suzuki, R.; Okuyama, T. A new phloroglucinol derivative from the brown alga Eisenia bicyclis: Potential for the effective treatment of diabetic complications. J. Nat. Prod. 2004, 67, 103–105.
- Jung, W.-K.; Heo, S.-J.; Jeon, Y.-J.; Lee, C.-M.; Park, Y.-M.; Byun, H.-G.; Choi, Y.H.; Park, S.-G.; Choi, I.-W. Inhibitory effects and molecular mechanism of dieckol isolated from marine brown alga on COX-2 and iNOS in microglial cells. J. Agric. Food Chem. 2009, 57, 4439–4446.
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins are polyphenolic metabolites of brown algae. Russ. J. Mar. Biol. 2018, 44, 263–273.
- Shibata, T.; Nagayama, K.; Tanaka, R.; Yamaguchi, K.; Nakamura, T. Inhibitory effects of brown algal phlorotannins on secretory phospholipase A2s, lipoxygenases and cyclooxygenases. J. Appl. Phycol. 2003, 15, 61–66.
- Kim, S.K.; Lee, D.Y.; Jung, W.K.; Kim, J.H.; Choi, I.H.; Park, S.G.; Seo, S.K.; Lee, S.W.; Lee, C.M.; Yea, S.S.; et al. Effects of Ecklonia cava ethanolic extracts on airway hyperresponsiveness and inflammation in a murine asthma model: Role of suppressor of cytokine signalling. Biomed. Pharm. 2007, 6, 289–296.
- Ah Jung, H.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206.
- Lee, S.-H.; Eom, S.-H.; Yoon, N.-Y.; Kim, M.-M.; Li, Y.-X.; Ha, S.-K.; Kim, S.-K. Fucofuroeckol-A from Eisenia bicyclis inhibits inflammation in lipopolysaccharide-induced mouse macrophages via downregulation of the MAPK/NF-κB signaling pathway. J. Chem. 2016, 2016, 6509212.
- Kim, M.-M.; Kim, S.-K. Effect of phloroglucinol on oxidative stress and inflammation. Food Chem. Toxicol. 2010, 48, 2925–2933.
- Shih, R.-H.; Wang, C.-Y.; Yang, C.-M. NF-κB signalling pathways in neurological inflammation: A mini review. Front. Mol. Neurosci. 2015, 8, 77.
- Yu, D.-K.; Lee, B.; Kwon, M.; Yoon, N.; Shin, T.; Kim, N.-G.; Choi, J.-S.; Kim, H.-R. Phlorofucofuroeckol B supresses inflammatory responses by down-regulating nuclear factor κB activation via Akt, ERK, and JNK in LPS-stimulated microglial cells. Int. Immunopharmacol. 2015, 28, 1068–1075.
- Kim, T.H.; Ku, S.-K.; Lee, T.; Bae, J.-S. Vascular barrier protective effects of phlorotannins on HMGB1-mediated proinflammatory responses in vitro and in vivo. Food Chem. Toxicol. 2012, 50, 2188–2195.
- Eom, S.-H.; Lee, E.-H.; Park, K.; Kwon, J.-Y.; Kim, P.-H.; Jung, W.-K.; Kim, Y.-M. Eckol from Eisenia bicyclis inhibits inflammation through the AKT/NF-Κb signaling in Propionibacterium acnes-induced human keratinocyte HACAT cells. J. Food Biochem. 2016, 41, e12312.
- Yang, Y.I.; Shin, H.C.; Kim, S.H.; Park, W.Y.; Lee, K.T.; Choi, J.H. 6,6’-Bieckol, isolated from marine alga Ecklonia cava, suppressed LPS-induced nitric oxide and PGE(2) production and inflammatory cytokine expression in macrophages: The inhibition of NF-kB. Int. Immunopharmacol. 2012, 12, 510–517.
- Sugiura, Y.; Usui, M.; Katsuzaki, H.; Imai, K.; Kakinuma, M.; Amano, H.; Miyata, M. Orally administered phlorotannins from Eisenia arborea supress chemical mediator release and cyclooxygenase-2 signaling to alleviate mouse ear swelling. Mar. Drugs 2018, 16, 267.
- Manzoor, Z.; Mathema, V.B.; Chae, D.; Kang, H.-K.; Yoo, E.-S.; Koh, Y.S. Octaphlorethol A inhibits the CpG-induced inflammatory response by attenuating the mitogen-activated protein kinase and NF-KB pathways. Biosci. Biotechnol. Biochem. 2013, 77, 1970–1972.
- Kim, T.H.; Lee, T.; Ku, S.-K.; Bae, J.-S. Vascular barrier protective effects of eckol and its derivatives. Biorg. Med. Chem. Lett. 2012, 22, 3710–3712.
- Stout, E.P.; Kubanek, J. Marine macroalgal natural products. In Comprehensive Natural Products II; Elsevier: Amsterdam, The Netherlands; Volume 2, Chapter 2.03; pp. 41–65.
- Mandrekar, V.K.; Gawas, U.B.; Majik, M.S. Brominated molecules from marine algae and their pharmacological importance. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands; Volume 61, Chapter 13; pp. 461–490.
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292.
- Katsui, N.; Suzuki, Y.; Irie, T. 5,6-Dibromoprotocatechualdehyde and 2,3-dibromo-4,5-dihydroxybenzyl methyl ether: New dibromophenols from Rhodomela larix. Tetrahedrn 1967, 23, 1185–1188.
- Wiemer, D.F.; Idler, D.D.; Fenical, W. Vidalols A and B, new anti-inflammatory bromophenols from the Caribbean marine red alga Vidalia obtusiloba. Cell. Mol. Life Sci. 1991, 47, 851–853.
- Kim, S.Y.; Kim, S.R.; Oh, M.J.; Jung, S.J.; Kang, S.Y. In vitro antiviral activity of red alga, Polysiphonia morrowii extract and its bromophenols against fish pathogenic infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus. J. Microbiol. 2011, 49, 102–106.
- Fan, X.; Xu, N.J.; Shi, J.G. Bromophenols from the red alga Rhodomela confervoides. J. Nat. Prod. 2003, 66, 455–458.
- Kang, N.J.; Han, S.C.; Kang, H.J.; Ko, G.; Yoon, W.J.; Kang, H.K.; Yoo, E.-S. Anti-inflammatory effect of 3-bromo-4,5-dihydroxybenzaldehyde, a component of Polysiphonia morrowii, in vivo and in vitro. Toxicol. Res. 2017, 33, 325–332.
- Ko, E.-Y.; Heo, S.-J.; Cho, S.-H.; Lee, W.W.; Kim, S.-Y.; Yang, H.-W.; Ahn, G.; Cha, S.-H.; Kwon, S.-H.; Jeong, M.S.; et al. 3-Bromo-5-(ethoxymethyl)-1,2-benzenediol inhibits LPS-induced pro-inflammatory responses by preventing ROS production and downregulating NF-κB in vitro and in a zebrafish model. Int. Immunopharmacol. 2019, 67, 98–105.
- Choi, Y.K.; Ye, B.-R.; Kim, E.-A.; Kim, J.; Kim, M.-S.; Lee, W.W.; Ahn, G.-N.; Kang, N.; Jung, W.-K.; Heo, S.-J. Bis (3-bromo-4,5-dihydroxybenzyl) ether, a novel bromophenol from the marine red alga Polysiphonia morrowii that suppresses LPS-induced inflammatory response by inhibiting ROS-mediated ERK signaling pathway in RAW 264.7 macrophages. Biomed. Pharmacother. 2018, 103, 1170–1177.
- Makkar, F.; Chakraborty, K. Highly oxygenated antioxidative 2H-chromen derivative from the red seaweed Gracilaria opuntia with pro-inflammatory cyclooxygenase and lipoxygenase inhibitory properties. Nat. Prod. Res. 2017, 32, 2756–2765.
- Makkar, F.; Chakraborty, K. Novel furanyl derivatives from the red seaweed Gracilaria opuntia with pharmacological activities using different in vitro models. Med. Chem. Res. 2018, 27, 1245–1259.
- Antony, T.; Chakraborty, K. First report of antioxidative 2H-chromenyl derivatives from the intertidal red seaweed Gracilaria salicornia as potential anti-inflammatory agents. Nat. Prod. Res. 2020, 34, 3470–3482.
- Kazłowska, K.; Hsu, T.; Hou, C.-C.; Yang, W.-C.; Tsai, G.-J. Anti-inflammatory properties of phenolic compounds and crude extract from Porphyra dentata. J. Ethnopharmacol. 2010, 128, 123–130.
- Jiang, M.; Wu, Z.; Guo, H.; Liu, L.; Chen, S. A review of terpenes from marine-derived fungi: 2015–2019. Mar. Drugs 2020, 18, 321.
- Ferdous, U.T.; Yusof, Z.N.B. Algal terpenoids: A potential source of antioxidants for cancer therapy. In Terpenes and Terpenoids—Recent Advances; Perveen, S., Al-Taweel, A.M., Eds.; IntechOpen: London, UK, 2020.
- Wijesinghe, W.A.J.P.; Kang, M.-C.; Lee, W.-W.; Lee, H.-W.; Kamada, T.; Vairappan, C.S.; Jeon, Y.-J. 5β-Hydroxypalisadin B isolated from red alga Laurencia snackeyi attenuates inflammatory response in lipopolysaccharide-stimulated RAW 264.7 macrophages. Algae 2014, 29, 333–341.
- Wijesinghe, W.; Kim, E.-A.; Kang, M.-C.; Lee, W.-W.; Lee, H.-S.; Vairappan, C.S.; Jeon, Y.-J. Assessment of anti-inflammatory effect of 5β-hydroxypalisadin B isolated from red seaweed Laurencia snackeyi in zebrafish embryo in vivo model. Environ. Toxicol. Pharmacol. 2014, 37, 110–117.
- Chatter, R.; Kladi, M.; Tarhouni, S.; Maatoug, R.; Kharrat, R.; Vagias, C.; Roussis, V. Neorogioltriol: A brominated diterpene with analgesic activity from Laurencia glandulifera. Phytochem. Lett. 2009, 2, 25–28.
- Daskalaki, M.G.; Vyrla, D.; Harizani, M.; Doxaki, C.; Eliopoulos, A.G.; Roussis, V.; Ioannou, E.; Tsatsanis, C.; Kampranis, S.C. Neorogioltriol and related diterpenes from the red alga Laurencia inhibit inflammatory bowel disease in mice by suppressing M1 and promoting M2-like macrophage responses. Mar. Drugs 2019, 17, 97.
- Antony, T.; Chakraborty, K. First report of antioxidant abeo-labdane type diterpenoid from intertidal red seaweed Gracilaria salicornia with 5-lipoxygenase inhibitory potential. Nat. Prod. Res. 2018, 34, 1409–1416.
- Bae, M.; Kim, M.-B.; Park, Y.-K.; Lee, J.-Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2020, 1865, 158618.
- Maeda, H.; Kanno, S.; Kodate, M.; Hosokawa, M.; Miyashita, K. Fucoxanthinol, metabolite of fucoxanthin, improve obesity-induced inflammation in a dipocyte cells. Mar. Drugs 2015, 13, 4799–4813.
- Miyahita, K.; Beppu, F.; Hosokawa, M.; Wang, S. Nutraceutical characteristics of the Brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364.
- Lin, J.; Huang, L.; Yu, J.; Xiang, S.; Wang, J.; Zhang, J.; Yan, X.; Cui, W.; He, S.; Wang, Q. Fucoxanthin, a marine carotenoid, reverses scopolamine-induced cognitive impairments in mice and inhibits acetylcholinesterase in vitro. Mar. Drugs 2016, 14, 67.
- Lin, J.; Yu, J.; Zhao, J.; Zhang, K.; Zheng, J.; Wang, J.; Huang, C.; Zhang, J.; Yan, X.; Gerwick, W.H.; et al. Fucoxanthin, a marine carotenoid, attenuates β-amyloid oligomer-induced neurotoxicity possibly via regulating the PI3K/Akt and the ERK pathways in SH-SY5Y cells Oxidative. Med. Cell. Longev. 2017, 2017, 6792543.
- Zhao, D.; Kwon, S.H.; Chun, Y.S.; Gu, M.Y.; Yang, H.O. Anti-neuroinflammatory effects of fucoxanthin via inhibition of Akt/NF-κB and MAPKs/AP-1 pathways and activation of PKA/CREB pathway in lipopolysaccharide-activated BV-2 microglial. Cells. Neurochem. Res. 2017, 42, 667–677.
- Jin, X.; Zhao, T.; Shi, D.; Ye, M.B.; Yi, Q. Protective role of fucoxanthin in diethylnitrosamine induced hepatocarcinogenesis in experimental adult rats. Drug Dev. Res. 2019, 80, 209–217.
- Rodriguez-Luna, A.; Avila-Roman, J. Fucoxanthin-containing cream prevents epidermal hyperplasia and UVB-induced skin erythema in mice. Mar. Drugs 2018, 16, 378.
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Oh, C.; Choi, Y.U.; Yoon, K.T.; Kang, D.-H.; Qian, Z.-J.; Choi, I.-W.; Jung, W.-K. Anti-inflammatory effect of fucoxanthin derivatives isolated from Sargassum siliquastrum in lipopolysaccharide-stimulated RAW 264.7 macrophage. Food Chem. Toxicol. 2012, 50, 3336–3342.
- Chengye, Z.; Daixing, Z.; Qiang, Z.; Shusheng, L. PGC-1-related coactivator (PRC) negatively regulates endothelial adhesion of monocytes via inhibition of NF-kB activity. Biochem. Biophys. Res. Commun. 2013, 439, 121–125.
- Ou, H.C.; Chou, W.C.; Chu, P.M.; Hsieh, P.L.; Hung, C.H.; Tsai, K.L. Fucoxanthin protects against oxLDL-induced endothelial damage via activating the AMPK-Akt-CREB-PGC1alpha pathway. Mol. Nutr. Food Res. 2019, 63, e1801353.
- Liu, M.; Li, W.; Chen, Y.; Wan, X.; Wang, J. Fucoxanthin: A promising compound for human inflammatory-related diseases. Life Sci. 2020, 255, 117850.
- Zheng, J.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Cha, J.W.; Hyun, J.W. Fucoxanthin enhances the level of reduced glutathione via the Nrf2-mediated pathway in human keratinocytes. Mar. Drugs 2014, 12, 4214–4230.
- Su, J.; Guo, K.; Huang, M.; Liu, Y.; Zhang, J.; Sun, L.; Li, D.; Pang, K.-L.; Wang, G.; Chen, L.; et al. Fucoxanthin, a marine xanthophyll isolated from Conticribra weissflogii ND-8: Preventive anti-inflammatory effect in a mouse model of sepsis. Front. Pharmacol. 2019, 10, 906.
- Wu, J.; Qin, Z.; Jiang, X.; Fang, D.; Lu, Z.; Zheng, L.; Zhao, J. ROS-responsive PPGF nanofiber membrane as a drug delivery system for long-term drug release in attenuation of osteoarthritis. NPJ Regen. Med. 2022, 7, 66.
- Meinita, M.D.N.; Harwanto, D.; Tirtawijaya, G.; Negara, B.F.S.P.; Sohn, J.-H.; Kim, J.-S.; Choi, J.-S. Fucosterol of Marine Macroalgae: Bioactivity, Safety and Toxicity on Organism. Mar. Drugs 2021, 19, 545.
- Hannana, M.A.; Sohag, A.; Al, M.; Dasha, R.; Haque, M.N.; Mohibbullahd, M.; Oktaviania, D.F.; Hossainb, M.T.; Choi, H.J.; Moon, I.S. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine 2020, 69, 153201.
- Abdul, Q.A.; Choi, R.J.; Jung, H.A.; Choi, J.S. Health benefit of fucosterol from marine algae: A review. J. Sci. Food Agric. 2016, 96, 1856–1866.
- Kirindage, K.G.I.S.; Jayasinghe, A.M.K.; Han, E.-J.; Jee, Y.; Kim, H.-J.; Do, S.G.; Fernando, I.P.S.; Ahn, G. Fucosterol isolated from dietary brown alga Sargassum horneri protects TNF-α/IFN-γ-stimulated human dermal fibroblasts via regulating Nrf2/HO-1 and NF-κB/MAPK pathways. Antioxidants 2022, 11, 1429.
- Kim, M.S.; Oh, G.H.; Kim, M.J.; Hwang, J.K. Fucosterol inhibits matrix metalloproteinase expression and promotes type-1 procollagen production in UVB-induced HaCaT cells. Photochem. Photobiol. 2013, 89, 911–918.
- Ying, R.; Zhang, Z.; Zhu, H.; Li, B.; Hou, H. The Protective Effect of Mycosporine-Like Amino Acids (MAAs) from Porphyra yezoensis in a Mouse Model of UV Irradiation-Induced Photoaging. Mar. Drugs 2019, 17, 470.
- Li, Y.; Li, X.; Liu, G.; Sun, R.; Wang, L.; Wang, J.; Wang, H. Fucosterol attenuates lipopolysaccharide-induced acute lung injury in mice. J. Surg. Res. 2015, 195, 515–521.
- Sun, Z.; Mohamed, M.A.A.; Park, S.Y.; Yi, T.H. Fucosterol protect cobalt chloride induced inflammatory by the inhibition of hypoxia-inducible factor through PI3K/Akt pathway. Int. Immunopharmacol. 2015, 29, 642–647.
- Mo, W.; Wang, C.; Li, J.; Chen, K.; Xia, Y.; Li, S.; Xu, L.; Lu, X.; Wang, W.; Guo, C. Fucosterol protects against concanavalin A-induced acute liver injury: Focus on P38 MAPK/NF-κB pathway activity. Gastroenterol. Res. Pract. 2018, 2018, 2824139.
- Wong, C.H.; Gan, S.Y.; Tan, S.C.; Gany, S.A.; Ying, T.; Gray, A.I.; Igoli, J.; Chan, E.W.L.; Phang, S.M. Fucosterol inhibits the cholinesterase activities and reduces the release of pro-inflammatory mediators in lipopolysaccharide and amyloid-induced microglial cells. J. Appl. Phycol. 2018, 30, 3261–3270.
- Fernando, I.P.S.; Jayawardena, T.U.; Kim, H.-S.; Lee, W.W.; Vas, A.P.J.P.; De Silva, H.I.C.; Abayaweera, G.S.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, D.-S.; et al. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh). Environ. Res. 2019, 171, 150–158.
- Güven, K.C.; Percot, A.; Sezik, E. Alkaloids in marine algae. Mar. Drugs 2010, 8, 269–284.
- Lunagariya, J.; Bhadja, P.; Zhong, S.; Vekariya, R.; Xu, S. Marine natural product bis-indole alkaloid caulerpin: Chemistry and biology, Mini-Rev. Med. Chem. 2019, 19.
- de Souza, E.T.; de Lira, D.P.; de Queiroz, A.C.; da Silva, D.J.C.; de Aquino, A.B.; Mella, E.A.C.; Lorenzo, V.P.; de Miranda, G.E.C.; de Araújo-Júnior, J.X.; Chaves, M.C.; et al. The antinociceptive and anti-Inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa. Mar. Drugs 2009, 7, 689.
- Lucena, A.M.M.; Souza, C.R.M.; Jales, J.T.; Guedes, P.M.M.; de Miranda, G.E.C.; de Moura, A.M.A.; Araújo-Júnior, J.X.; Nascimento, G.J.; Scortecci, K.C.; Santos, B.V.O.; et al. The bisindole alkaloid caulerpin, from seaweeds of the genus Caulerpa, attenuated colon damage in murine colitis model. Mar. Drugs 2018, 16, 318.
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Naidu, K.A.; Shang, X.; Keum, Y.-S. Omega-3 polyunsaturated fatty acids (PUFAs): Emerging plant and microbial sources, oxidative stability, bioavailability, and health benefits—A Review. Antioxidants 2021, 10, 1627.
- Calder, P.C. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochim 2009, 91, 791–795.
- Giacobbe, J.; Benoiton, B.; Zunszain, P.; Pariante, C.M.; Borsini, A. The anti-inflammatory role of omega-3 polyunsaturated fatty acids metabolites in pre-clinical models of psychiatric, neurodegenerative, and neurological disorders. Front. Psychiatry 2020, 11, 122.
- Van Ginneken, V.J.T.; Helsper, J.P.F.G.; de Visser, W.; van Keulen, H.; Brandenburg, W.A. Polyunsaturated fatty acids in various macroalgal species from north Atlantic and tropical seas. Lipids Health Dis. 2011, 10, 104.
- Jaswir, I.; Hammed, A.M. Anti-inflammatory compounds of macroalgae origin: A review. J. Med. Plants Res. 2011, 5, 7146–7154.
- Arita, M.; Clish, C.B.; Serhan, C.N. The contributions of aspirin and microbial oxygenase to the biosynthesis of anti-inflammatory resolvins: Novel oxygenase products from x-3 polyunsaturated fatty acids. Biochem. Biophys. Res. Commun. 2005, 338, 149–157.
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505.
More
