Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Nabilah Saafie and Version 2 by Lindsay Dong.
Rapid urban and industrial sectors generate massive amounts of wastewater, creating severe ecological disruption and harming living organisms. The number of harmful pollutants such as dyes, heavy metals, antibiotics, phenolic compounds, and volatile and several organic chemicals discharged into aquatic systems varies depending on the effluent composition of various sectors. MXene-based composites with unique characteristics were spotlighted as newly developed nanomaterials specifically for environmental-related applications.
- MXene-based catalysts
- photocatalyst
- wastewater treatment
1. Introduction
1.1. Background Study of MXene
In 2011, 2D-layered titanium carbide powder (Ti3C2Tx) was the first candidate of the MXene family introduced by Naguib et al. from Drexel University. MXene has a formula of Mn+1XnTx, which is fabricated by etching MAX phase (Mn+1AXn) in which M corresponds to transition metal, X denotes carbon/nitrogen, T indicates the functional groups such as hydroxyl, oxygen, or fluorine, A represents elements from the group, and n is an integer. MXene materials were initially investigated as a promising alternative for electrochemical energy storage in batteries and super capacitors due to their high electrical conductivity, structural stability, and hydrophilicity [1][7]. In 2011, 2D-layered titanium carbide powder (Ti3C2Tx) was the first candidate of the MXene family introduced by Naguib et al. from Drexel University [2][8]. MXene materials were broadly examined as vehicles for energy storage/delivery in devices such as Li/Na-ion batteries and supercapacitors, owing to their lamellar structures and exceptional electrical conductivity, in 2012 [3][9]. MXene was then used to remove various pollutants from the environment, including dye in 2014 [4][10], Cr (VI) in 2015 [5][11], U(VI) in 2016 [6][12], and N2 reduction in 2018 [7][13]. In recent developments, MXene has also been recommended for use as a negative electrode in acidic electrolytic solutions to establish higher concentration gradients and increase energy density [8][14]. Metallic MXenes have the potential to be tuned to match the semiconductor’s band edge by choosing specific compositions and managing their surface terminations, making them promising electrode materials for nanoelectronics devices.
1.2. Basic Principle of MXene
MXene belongs to transition metal, carbides, and nitrides with an uneven distribution of functional groups. MXene has a formula of Mn+1XnTx fabricated by etching MAX phase (Mn+1AXn) in which M corresponds to transition metal, X denotes carbon/nitrogen, T indicates the functional groups such as hydroxyl, oxygen or fluorine, A represents an element from the group, and n is an integer. Various functional groups of oxygen (-O), fluorine (F), and hydroxyl (-OH) were molded onto MXene surfaces. They were recognized using energy-dispersive X-ray spectroscopy (EDX) to detect the elemental compositions. The intralayer skeleton, interlayer, and surface terminating groups area make up the structure of MXene, Ti3C2. As confirmed by neutron diffraction, Ti is bonded to C via an ionic bond in the interlayer skeleton between the Van der Waals forces and hydrogen bonding between -O or F atoms on the surface [9][21]. MXene materials have gained significant attention for their wide variety of compositions, conductive properties, and unique morphology. The MXene compound possesses superior reflectivity in the ultraviolet (UV) light absorptions with the reported region from a wavelength between 300 and 500 nm, which was a crucial property for photocatalytic reactions with energy gaps of about 0.25–2.0 eV [10][22]. Moreover, the lattice layered structure of surface functionalized MXene allows for highly competent and robust photocatalysts with nitride and carbide bonding–this leads to an efficient generation of the reactive species responsible for pollutant degradation. In addition, MXene owns the hydrophilic nature incorporated with the active functional group on its surface to tackle ionic/molecular species, especially for environmental remediation. Furthermore, the morphology of the MXene flake’s structure was identified by some characterization methods, such as transmission electron microscopy (TEM) and scanning electron microscopy (SEM), which displayed accordion-like multilayer structures [11][12][13][23,24,25]. The most well-known 2D materials are graphene-based nanomaterials. Still, they only have a single atomic carbon layer which limits their application, whereas MXene may reach three, five, or seven atomic layers. The inter-planar distance of MXene sheets was about 0.242 nm with the (1 0 3) plane of MXene.2. Synthesis Routes of MXene
The introduction of MXene was first synthesized by etching an A layer from a MAX phase with the HF or HCl-LiF method. The enhancement of the hydrophilic compound due to the functionalization of MXene was the result of the etching method. The multilayered MXene is held weakly together by van der Waals bonds and hydrogen. Congruously, HCl and LiF solution’s etching method gives larger interlayer spacing than HF because of the expansion of hydrated Li-ion intercalation and chloride termination. Conversely, HCl–LiF is a lower fluorine content solution that creates a safer and easier method. However, the latter method’s concentration optimization of these two ingredients, LiF and HCl, improved the quality of the produced MXene [14][28]. Additionally, based on Khatun and Roy’s optimization analysis, 24 h of etching produces well-interspaced accordion-like multilayer MXene, and shorter etching times are insufficient to remove the ideal amount of Al. On the other hand, 48 h of etching causes pits to form and flakes to break, which weakens the MXene’s properties [15][29]. Various fluoride-free etching methods have been developed to fabricate MXene to reduce its adverse effect on the environment, such as alkali-based etching, Lewis acid etching, and electrochemical etching (E-etching). There are a few reports on using alkaline etchants as fluorine-free synthetic methods. In the first work by Li et al. [16][39], an alkali-assisted hydrothermal method which inspired the Bayer process was used to prepare M-MXene. It was found that the Al from Ti3AlC2 was successfully removed at 270 ˚C in a 27.5 M NaOH solution for 12 h. Ideally, Al layers were attacked by OH; thus, Ti and Al were terminated with −OH and −O. As a result, this process yields a 92 wt. % multilayer Ti3C2Tx with an interlayer spacing of 1.2 nm. Granting this etching technique prevents the formation of −F terminal groups. Conversely, the high concentrations required of base and harsh reaction conditions are still dangerous and unsuitable for large-scale synthesis [17][40]. The Lewis acidic etching mechanism is proposed to etch MAX phases by direct redox coupling between the A element and the cation of Lewis acid molten salt [8][14].3. Environmental Applications
3.1. Photocatalysis Mechanism
The photocatalysis system mainly depends upon light energy or wavelengths and a photocatalyst. The semiconductors are generally utilized as photocatalysts, and their electronic structures could be helpful to serve as sensitizers for light irradiation [18][49]. In the degradation mechanism, the light source falls over the photocatalyst surface, and if this energy is equal to or greater than photocatalyst band gap energy, then electrons in the VB are disturbed and transferred towards the CB of the photocatalyst, leaving the holes in the VB. VB holes may oxidize donor molecules and generate hydroxyl radicals after a reaction with water molecules. On the other hand, electrons in CB produce superoxide radicals after a reaction with oxygen species. The produced holes and electrons may cause oxidation and reduction reaction processes that can be adsorbed over the photocatalyst surface to provide necessary outputs [19][50]. Similar photocatalysis mechanisms have been proposed in several studies. Vishal et al. proposed a photocatalysis mechanism using ZnO/Bi2WO6/M-MXene photocatalyst for ciprofloxacin under a visible-light system. Bi2WO6 and ZnO are activated under UV and visible-light systems, separating photogenerated electrons and holes. Photoelectrons from ZnO move towards the CB of Bi2WO6, and then these electrons are transferred into the CB of MXene. Firstly, MXene carried the photoelectrons towards active sites owing to its good metallic structure and produced H2O2 after reacting with adsorbed oxygen molecules. Furthermore, Bi2WO6 and ZnO also reacted with adsorbed molecules to produce H2O2 species, resulting in the generation of hydroxyl radicals. Secondly, holes in the VB of Bi2WO6 transferred towards ZnO VB and produced hydroxyl radicals after reacting with adsorbed water molecules that degrade ciprofloxacin pollutants [20][51]. In another study, TiO2/M-MXene photocatalyst was used to propose a photocatalysis mechanism for RhB. The photoelectrons in TiO2 move from VB towards CB, leaving holes in VB and producing photoelectrons and hole-pairs [21][52]. Similar photocatalysis mechanisms have also been investigated in other studies using TiO2/M-MXene for carbamazepine [22][53], CuFe2O4/M-MXene for sulfonamides [23][54], BLFMO-5/M-MXene hybrid for cango red [24][55], g-C3N4/S-MXene for RhB [25][56], CoO@TiO2/M-MXene, and Fe(Co)/M-MXene/ZSM-5 for phenol [26][27][57,58].3.2. Photocatalysis
The photocatalytic degradation of antibiotics and other different organic pollutants from wastewater has been considered the most effective method, owing to its simplicity, environment-friendliness, and short time-consuming nature [28][29][30][59,60,61]. The development of antimicrobial resistance has appeared as a significant health problem for humans and other living creatures and the ecosystem disturbances in a natural water environment. The fast recombination of photo charge carriers’ efficiency and stability of a single photocatalyst minimizes its photo performance. Therefore, MXene assembled a co-catalyst to effectively minimize the photo charge carrier recombination effect and improve the photocatalytic power. Until now, several MXene and MXene-based photocatalysts have shown efficient photocatalytic performance for the remediation of different organic compounds from wastewater. Furthermore, previous studies have found that the combination of MXene and other co-catalysts provides abundant active sites and oxygen-containing functional groups that respond to the magnetic field. The magnetic field could boost the catalyst reactivity and enhance the degradation performance [31][64]. MXene-based photocatalysts have abundantly been employed to eliminate different types of dyes. The development of Z-scheme heterojunction photocatalysts could be an excellent photocatalytic agent. In this regard, Tu et al. prepared g-C3N4/S-MXene photocatalyst through a one-step H2O2 oxidation process. The development of Z-scheme heterojunction between MXene and g-C3N4 hybrid could have a solid power for a separate photogenerated recombination rate and is effective for the degradation of RhB and tetracycline up to 97.22 and 86.34%, respectively, in 60 min [25][56].3.3. Adsorption Mechanism
In the adsorption mechanism, the adsorbate is strongly attributed to the surface of employed adsorbent until it has attained the equilibrium stage. The adsorption mechanism contains several significant steps such as (a) physical adsorption: settlement of adsorbate occurred over the surface of applied adsorbents, (b) complexation and precipitation: deposition of adsorbate occurred over the surface of the applied adsorbent, and (c) pore filling: condensation of adsorbate inside the pore of adsorbent. The adsorption mechanism has been defined in literature by employing different kinetic models. From the several kinetics equations, pseudo-first order, pseudo-second order, Elovich, and intraparticle diffusion kinetic model, equations were selected to understand the adsorption mechanism [32][78]. The adsorption mechanism could be investigated more frequently through adsorption equilibrium studies by employing different isotherm models. The equilibrium relationship between adsorbate and adsorbent can be described using several isotherm equations that quantify the solute amount at room temperature. Langmuir, Freundlich, and Temkin isotherms have been the most commonly used models to produce isotherm profiles based on experimental results [30][33][61,85].3.4. Adsorption
The adsorption process provides reasonable compensations such as safe operations, recycling and remediation to the required concentration range. Moreover, the adsorption technique can provide an excellent removal rate for it to be commercially viable [34][35][76,86]. Due to the rapid development of industrialization, dye wastewater originating from textile, paper, and printing activities affected the ecological environment and human health. Dye pollutants are a family of organic compounds with high mobility, substantial toxicity, and poor biodegradability [36][1]. The aromatic molecular makeup of dyes were reported as cancer-causing and mutagenic, whereas the metal-containing dyes can cause severe renal system damage [4][10]. Many strategies were attempted to address this issue, primarily through adsorption, a potentially promising technique due to the easy operation and economical and energy saving [37][89]. Therefore, an MXene-based catalyst is a suitable choice to treat dye-polluted wastewater due to the surface functional group structures, effectively absorbing and activating the dye during adsorption activity.3.5. Water Splitting Mechanism
The photocatalytic process is a chemical reaction that can be encouraged through photoirradiation under a suitable photocatalyst material. This kind of photocatalyst will have a solid ability to improve the chemical reaction without being used or converted into another phase. The basic working principle of the photocatalytic mechanism for H2 production via water splitting is simple. Initially, when light with energy more significant than the band gap of the photocatalyst is irradiated, the empty conduction band (CB) and the filled valence band (VB) are separated. The electrons in the VB are excited by the CB, and the electrons (e−) and hole pairs (h+) are generated. These e− and h+ reduce and oxidize the chemicals over the photocatalyst surface, respectively, unless they recombine to avoid clean chemical reactions. When the same amount of e− and h+ is used for a chemical reaction and recombination, the photocatalyst’s original structure (or chemical composition) does not change. Recently, several MXene-based photocatalysts have been proposed for photochemical reaction mechanisms to produce H2 under a visible-light activation system. Molybdenum@TiO2@M-MXene photocatalysts have effectively transformed and separated photoinduced electrons and hole pairs under a visible-light system. In the photocatalytic mechanism, a maximum number of photogenerated electrons inside the CB of TiO2 may quickly migrate towards the MXene side MoS2 impurity bandgap caused by molybdenum vacancies, resulting in an electron-rich environment over the surface planes of MoS2 and Mxene, via which H2 is produced through the reduction of water molecules [38][101].3.6. Water Splitting
In addition to the versatile photocatalytic decomposition of pollutants, MXene-based catalysts significantly contribute to sustainable clean energy production. Environmental contamination and the energy crisis were global issues due to the rapid consumption of fossil fuels. Fossil fuel reserve depletion times for oil, coal, and gas are approximately 35, 107, and 37 years, respectively, and are computed by the modified formula from the Klass model [39][108]. Therefore, photocatalytic water-splitting technologies are an excellent way to replace fossil fuels such as hydrogen and oxygen. MXene has the potential as a promising candidate for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) due to its unique features: (1) excellent metal conductivity, (2) higher hydrophilic functionalities, and (3) significant redox reactivity [13][25].4. MXene-Based Nanomaterials
MXene-based photocatalysts are created by combining an MXene photocatalyst with another type of photocatalyst or co-catalyst [40][126]. Improved space charge transfer and separation can be achieved by combining MXene with semiconductors and selecting optimal CB and VB potentials. These nanostructures include 0D/2D, 1D/2D, 2D/2D heterostructures, and other combinations. The band alignment of an MXene-based photocatalyst with a different type of photocatalyst can be tuned to one of three different types. There are three varieties of heterostructure band alignment: Type I (straddling gap), Type II (staggered gap), and Type III (broken gap) [41][127]. The CB and VB are combined in one layer on Type I, resulting in a straddling band alignment. As a result, when the linked photocatalyst absorbs photon energy equal to or greater than its band gap energy, electron-hole pairs in a single component within the heterostructure photocatalyst separate and migrate [42][128]. As a result, because the CB and VB of component B are at a lower and greater energy than component A, electrons and holes will be transported and accumulate on component A. Two separate CB and VB monolayers contribute to the Type II heterostructure, resulting in upward or downward band bending [43][70].4.1. MXene/TiO
2
Composites
Titania, TiO2 was the most favourable and environmentally friendly promising photocatalyst, with a high photocatalytic activity that is closely related to its lattice structure. In this regard, this photocatalyst was capable of photoactivity reaction, a solid oxidising power, and high chemical/photo-stability. In the tetragonal unit cell of anatase, each titanium Ti atom is surrounded by six octahedra oxygen, O atoms. Although there are advantages such as a high carrier-forming capacity, toxicity, and good stability, TiO2 charge carriers tend to show rapid rearrangement and poor performance in visible light owing to their considerable band gap energy, 3.2 eV [44][133]. Anatase TiO2 also absorbs very little sunlight in the UV region (approximately 5%) owing to its considerable band gap energy [10][22].4.2. MXene/g-C
3
N
4
Composites
Graphitic carbon nitride (GCN), g-C3N4, is a metal-free photocatalyst with a medium band-gap energy of 2.7 eV, [45][141]. The polymeric nature of g-C3N4 was structurally analogous to graphene, which was considered the most stable C3N4 type at ambient conditions. In addition, this photocatalyst is also highly resistant to thermal and chemical environments, making it a stable material. Furthermore, this nature allows for multiple excitations from a single photon’s absorption, thus effectively producing reactive species for pollutant degradation. Ideally, the compositions of g-C3N4, consisting only of carbon and nitrogen atoms were reported with a C/N molar ratio of 0.75 [46][142]. In addition, g-C3N4 has a surface termination as defects and nitrogen atoms for electron localization. It has a unique microstructure in which anchoring inorganic and organic functional groups act as active sites [47][143]. Conversely, g-C3N4 suffers from easy recombination of photo-generated electron-hole pairs, which limits its sorption capacity due to the low specific surface area and quantum efficiency [48][144]. Moreover, the non-utilization of visible light (450 nm) and low efficiency of charge separation and transfer of g-C3N4 itself restrict its further applications. To overcome the bulk individual g-C3N4, more light collectors and charge transfer tunnels need to implement in the photocatalyst composites to boost their photocatalytic activity [49][145].4.3. MXene/BiVO
4
Composites
Bismuth vanadate (BiVO4) is a photocatalyst material with various intrinsic features such as a suitable band gap, proper band location, excellent stability, and a friendly environment. Photocatalytic properties of BiVO4 are strongly dependent on its crystal structure, which originates from the mineral pucherite in an orthorhombic structure [50][154]. Its improved photocatalytic performance was due to the better optical absorption achieved with a monoclinic scheelite structure with a band gap energy of 2.4 eV compared to a tetragonal structure with 2.9–3.1 eV [51][155]. This is the most significant photocatalyst for visible light water oxidation and photodegradation due to the narrow band gap, which can effectively oxidize water to release oxygen and generate active substances [52][156]. Primarily, BiVO4-based materials were extensively used for toxic pigments in the coating and plastic industry and have been shifted into photocatalytic applications due to their intrinsic crystal structure [53][157]. Nevertheless, the position of the conduction band of BiVO4 (ECB = 0.3 eV) in a more favourable spot severely limits the reduction capacity of BiVO4 and constraints in photocatalytic environmental remediation application [54][158]. Consequently, modest photocatalytic performance was observed in pristine BiVO4 due to the low electrical conductivity. On the other hand, slow water oxidation kinetics of BiVO4 occur due to the photogenerated holes on the photocatalyst surface, promoting the fast recombination of electron-hole pairs [41][127]. Herein, by modifying BiVO4 photocatalysts with MXene by in-situ engineered heterojunctions, the hybrid composite system can control the charge transfer process. Co-catalyst modification was also a primary fashion to accelerate surface redox kinetics. Therefore, the photo-generated holes migrated to the surface of MXene and generated an oxidising reaction.5. Multi-Roles of MXene-Based Nanomaterials
5.1. Photocharge Separation and Transfer Role
In photocatalysis, improving the separation efficiency of photogenerated charge carriers has been a hot research topic. MXene, as a co-catalyst, could prevent photogenerated electrons and holes from recombination in the photocatalytic system. The Schottky junction might be experimentally established between semiconductor photocatalysts and MXene [55][163]. According to theoretical estimates, electrons could move from g-C3N4 to MXene in their composites due to the difference in their Fermi levels (EF) and the development of Schottky junctions. Furthermore, because MXene showed strong metallic conductivity and favourable H adsorption-free energy, H+ may be converted to H2, resulting in a significantly higher H2 production. Ag3PO4 has a high carrier recombination rate during the photocatalytic process and is readily converted to elemental silver. In light of this, a Schottky junction was built, and MXene was introduced as a co-catalyst to improve the photocatalytic performance and stability of Ag3PO4. Surprisingly, after eight cycles, the photocatalytic clearance rate of tetracycline hydrochloride solution (TC-H) over the Ag3PO4/MXene composite marginally decreased, indicating improved anti-photo corrosion efficacy and photocatalytic activity.5.2. Photocatalytic Active Sites
The surface characteristics of materials are widely recognized to significantly impact their adsorption and photocatalytic activities. MXene and MXene-based materials have 2D heterostructures that increase the contact area with other 0D, 1D, and 2D materials and provide more surface reaction sites for photocatalysis applications. MXene etched using HF solution typically has a large number of −F terminations, but those etched with a mix of HCl and LiF have a large number of −O terminations. Ran et al. studied the influence of surface terminations on the photocatalytic property of MXene composites. They found that changing −F to −O/−OH enhanced the number of active sites, enhancing the photocatalytic H2 evolution [56][164].6. Economic and Eco-Friendly Feasibility
MXene-based nanomaterials are attracting researchers’ interest for use in various disciplines due to their low cost of manufacturing and use and environmental friendliness. Figure 11 depicts MXene-based nanomaterials’ use to remove several contaminants. Different dyes such as methylene blue (MB), saffranine T (ST), neutral red (NR), antibiotics, heavy metals, and phenolic compounds are hazardous to the atmosphere and community. MXene-based nanomaterials’ active surface area, layered structure, and charged surface aid in removing hazardous substances from aqueous solutions. Several heavy metals, such as Pb(II), Cu(II), Cd (II), Cr(IV), and Hg(II), are inorganic and organic chemicals that are difficult to break down and accumulate in living things. MXene-based nanoparticles are used in this application because they include oxygen-containing groups and have a more extensive surface area for removing irons from effluent perfectly. Human respiratory infection and other health problems are caused by wastewater and certain volatile organic chemicals. MXene-based nanomaterials’ features, such as high adsorption energy and selectivity, help to remove toxic organic chemicals and hazardous water pollutants. Pu ions, uranium (II), and Neptune (Np) are radioactive constituents that are radiologically and chemically hazardous. They pollute the environment with nuclear waste. MXene-based nanomaterials are used to eliminate these radioactive materials because of their remarkable ability to resist higher radiation and good chemical compatibility. MXene-based nanomaterials also remove contaminants such as phosphates in the water, phenol, and highly charged cations, owing to their active surface morphology.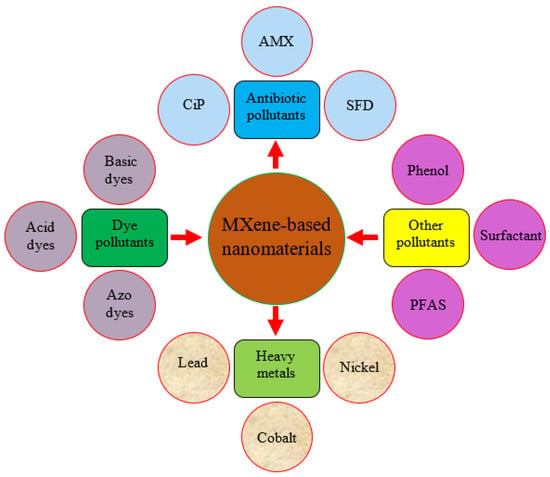
Figure 11.
Applications of MXene-based nanomaterials for the remediation of different pollutants from wastewater.
