You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Wei Zhang.
Arsenic is an element that is distributed globally and is abundant in the Earth’s crust (20th most abundant element) and seawater (14th most abundant element). It is classified as a metalloid and exhibits both metallic and non-metallic properties. The high solubility and mobility of arsenic in aquatic environments affects its global cycling. Furthermore, the biological processes in the aquatic environment are discussed, especially the possible microbe-mediated reactions of arsenic in sediments. In addition, various environmental factors, such as redox conditions, pH, and salinity, which influence the transformation of arsenic species.
- arsenic occurrence
- arsenic speciation
- arsenic aquatic concentrations
1. Introduction
Arsenic has been introduced into aquatic environments through both natural and anthropogenic sources [22,23][1][2]. Natural sources include rock weathering and geothermal activities [24][3], with global arsenic emissions of ~12,000 t/y [25][4]. Simultaneously, human activities, such as metal mining and smelting, the burning of fossil fuels, and the discharge of arsenic-based industrial wastes lead to significant amounts of arsenic entering the environment [22][1]. Approximately 82,000 t of arsenic is emitted into the environment each year from anthropogenic sources [26][5]. Arsenic pollution of aquatic environments has attracted worldwide attention. Some water bodies contaminated with arsenic by natural and human activities have significant concentrations, which endanger the safety of living organisms and consumers. Arsenic from natural and anthropogenic sources eventually converges in the environment through biogeochemical cycles, including weathering reactions, atmospheric settlement, and biological activity (Figure 1). Therefore, in environmental risk evaluations of arsenic, both natural and anthropogenic sources should be considered.
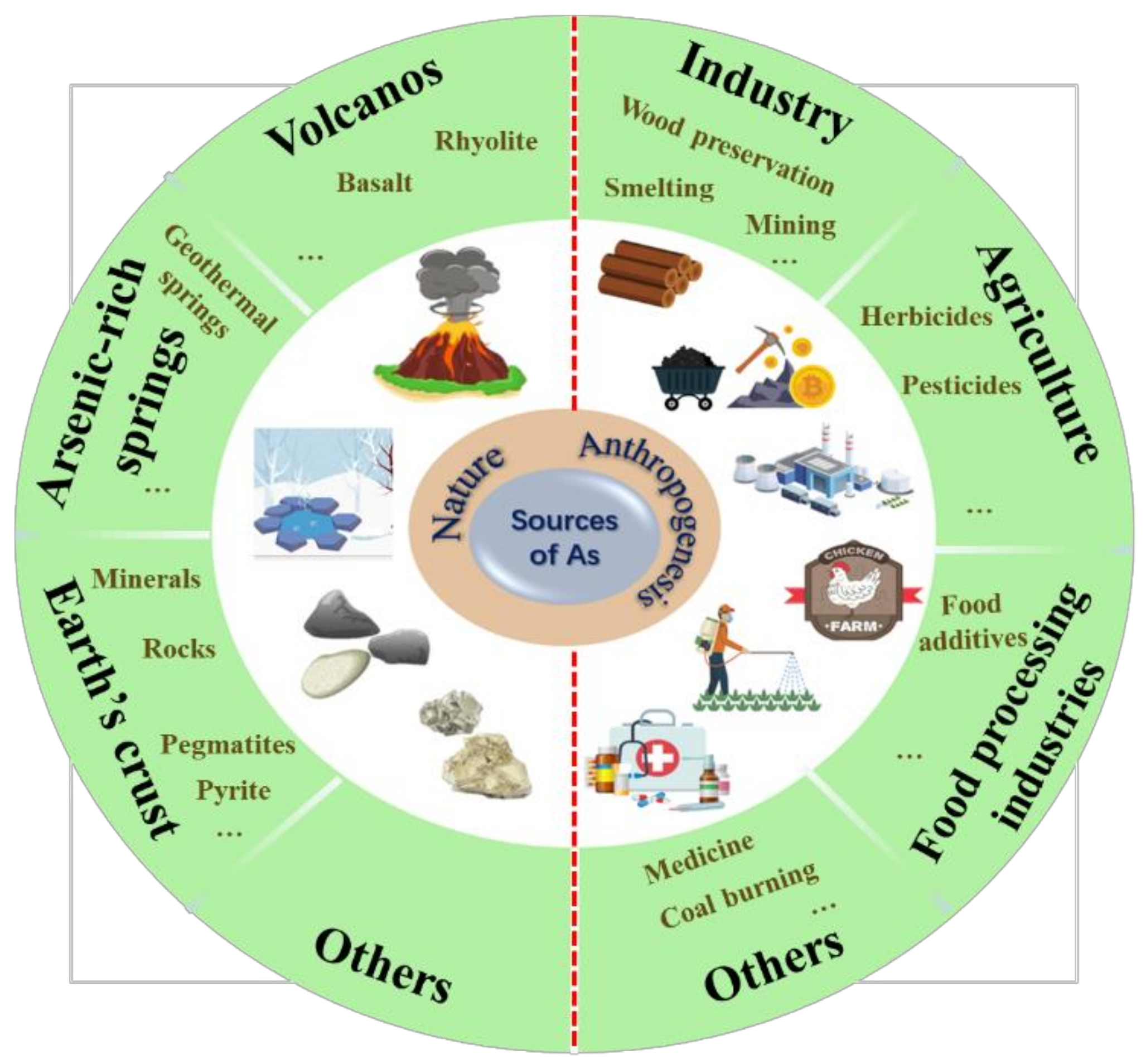
Figure 1.
Sources of arsenic pollution in aquatic environments.
2. Natural Sources of Arsenic
Natural sources refer to arsenic entering aquatic environments through natural geological processes, with rock weathering being the principal natural method [20][6]. Weathering results in elevated concentrations of arsenic in natural waters, especially in groundwater [22][1]. Arsenic of geological origin is found in all three rock types: igneous, metamorphic, and sedimentary rocks [27][7]. The arsenic concentrations in different igneous rocks tend to be comparable and low. In most metamorphic rocks, arsenic concentrations are similar (≤5 mg/kg), except in pelitic rocks, such as slates and phyllites, which have high arsenic concentrations of approximately 18 mg/kg [28][8]. Compared with the aforementioned two rock types, relatively higher arsenic concentrations, between 5 and 10 mg/kg, are found in sedimentary rocks. Among these, argillaceous deposits, which contain more binding matter, have been found to have a wider distribution with higher arsenic concentrations [23][2]. For instance, the highest concentration of arsenic (35,000 mg/kg) was found in some coal samples [29][9]. Arsenic in the oceanic lithosphere was also a natural source for the seawater, but the data and information on arsenic concentration were rare [30][10]. It was reported that arsenic concentrations ranged from 0.5 to 5.8 mg/kg in magmatic rocks from island arc and back-arc settings, which were slightly higher than basic and ultrabasic rocks [31][11].
Geothermal activity is another major natural source of arsenic contamination [32][12], with arsenic appearing frequently in geothermal fluids [33,34][13][14]. It has been demonstrated that arsenic may leach from rock or molten lava [33][13]. Geothermal waters, which commonly contain elevated arsenic concentrations mixed with drinking water sources, give rise to arsenic contamination, as reported in a geothermal field in Mexico, where the highest recorded arsenic concentration was 73,600 μg/L [35][15]. Arsenic can leach from volcanic rocks into the surrounding groundwater; in California, for example, the arsenic concentration in volcano-related groundwater is approximately 8000 μg/L [16]. Hot springs associated with volcanogenic sources carry arsenic from the mantle to the Earth’s surface [14][17]. The concentration of arsenic in the hot springs of geothermal fields has been reported to range from 170 to 4800 μg/L in New Zealand [26][5]. For the marine environment, hydrothermal fluids with variable amounts of arsenic were an important input source of arsenic [30][10]. Overall, arsenic discharged into the aquatic environment through natural pathways constitutes the background arsenic concentration in the aquatic environment.
3. Anthropogenic Sources of Arsenic
Anthropogenic sources refer to the release of arsenic-containing waste generated by human activities, including industrial and agricultural activities and domestic sewage, into rivers, oceans, and municipal water supplies [7,14,36][17][18][19]. Anthropogenic activities have led to significant levels of arsenic contamination in surrounding environments, with industrial activities being the most notable. Industrial processes, such as mining, smelting, ore beneficiation (tin, zinc, copper, and gold), and wood preservation, all contribute to an increase in the arsenic content of industrial wastewater [37][20]. In terms of marine environment alone, the major anthropogenic sources of arsenic in surface seawaters are riverine inputs, which are contaminated by various pollutants such as industrial and agricultural chemicals [23][2]. It has long been recognized that mining, smelting of nonferrous metals, and metal ore processing are major sources of arsenic contamination worldwide. Arsenic pollution events associated with mining activities have been highlighted in numerous studies [16,38,39][16][21][22]. High concentrations of arsenic have frequently been reported to reach 48,000 mg/L [16]. In addition, the nonferrous metallurgical industry emits more than 40,000 tons of arsenic each year [40,41][23][24]. At present, the disposal of smelting and mining waste has led to arsenic pollution in groundwater in many places, including the western parts of the USA, southeastern Europe, Canada, Chile, Ghana, South Africa, Thailand, and Turkey [22,42,43][1][25][26]. Electrical waste (semiconductors) [44][27] and chemical products [45][28] also contribute to industrial sources of arsenic.
Agricultural wastewater is another major anthropogenic source of arsenic. For hundreds of years, inorganic arsenic has been widely used in herbicides, pesticides, insecticides, and fungicides [46,47,48][29][30][31]. In crop cultivation, arsenic has been widely used in pesticides and herbicides since the late 1800s. Widespread usage of inorganic arsenic compounds, mainly sodium arsenite, began in the early 1900s. Consequently, organic arsenic is used as a pesticide to control insect pests in various crops (tobacco, cotton, and potatoes) and fruit trees [43][26]. Zinc methyl arsenate is used to prevent infestation by Corticium sasakii in rice paddies [31][11]. In the late 1980s, legislation prohibited the use of inorganic arsenic-based pesticides in agriculture. However, organic arsenicals, such as MMA and DMA, are still allowed owing to their lower toxicity [23][2]. Arsenide is widely used in wood preservation and as a food additive. For example, 4-aminoben-zenearsenic acid and 3-nitro-4-hydroxyphenylarsonic acid (roxarsone) are additives in animal food used to raise broiler chickens and cause weight gain during chicken breeding, which leads to a high arsenic content in poultry excrement [49][32]. The maximum arsenic content in poultry litter was 40 µg/g [50][33]. This arsenic-bearing poultry litter is often used as fertilizer for farmlands and pastures. Arsenic in fertilizer is mostly water soluble and may enter the aquatic environment via biogeochemical cycles [51][34]. In summary, there are many possible routes for arsenic transfer into the aquatic environment from both natural and anthropogenic sources.
References
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Geochem. 2002, 17, 517–568.
- Garelick, H.; Jones, H.; Dybowska, A.; Valsami-Jones, E. Arsenic pollution sources. Rev. Environ. Contam. Toxicol. 2008, 197, 18–51.
- Moriarty, M.M.; Lai, V.W.M.; Koch, I.; Cui, L.; Combs, C.; Krupp, E.M.; Feldmann, J.; Cullen, W.R.; Reimer, K.J. Speciation and toxicity of arsenic in mining-affected lake sediments in the Quinsam watershed, British Columbia. Sci. Total Environ. 2014, 466, 90–99.
- Boyle, R.W.; Jonasson, I.R. The geochemistry of arsenic and its use as an indicator element in geochemical prospecting. J. Geochem. Explor. 1973, 2, 251–296.
- Janga, Y.C.; Somannaa, Y.; Kimb, H. Source, Distribution, Toxicity and Remediation of Arsenic in the Environment–A review. Int. J. Appl. Environ. Sci. 2016, 11, 559–581.
- Ferguson, J.F.; Gavis, J. A review of the arsenic cycle in natural waters. Water. Res. 1972, 6, 1259–1274.
- Wang, J.; Sun, X.; Xing, Y.; Xia, J.; Feng, X. Immobilization of mercury and arsenic in a mine tailing from a typical Carlin-type gold mining site in southwestern part of China. Int. J. Appl. Environ. S 2016, 11, 559–581.
- Goldschmidt, V.M. Geochemistry; Oxford University Press: Oxford, UK, 1954.
- Belkin, H.E.; Zheng, B.; Zhou, D.; Finkelman, R.B. Chronic Arsenic Poisoning from Domestic Combustion of Coal in Rural China: A Case Study of the Relationship between Earth Materials and Human Health. In Environmental Geochemistry; Elsevier: Amsterdam, The Netherlands, 2008; pp. 401–420.
- Breuer, C.; Pichler, T. Arsenic in marine hydrothermal fluids. Chem. Geol. 2013, 348, 2–14.
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta: Int. J. Pure Appl. Anal. Chem. 2002, 58, 201–235.
- Tamaki, S.; Frankenberger, W.T. Environmental biochemistry of arsenic. Rev. Environ. Contam. T 1992, 124, 79–110.
- Ballantyne, J.M.; Moore, J.N. Arsenic geochemistry in geothermal systems. Geochim. Cosmochim. Acta 1988, 52, 475–483.
- White, D.E. Magmatic, connate, and metamorphic waters. Geol. Soc. Am. Bull. 1957, 68, 1659–1682.
- Birkle, P.; Bundschuh, J.; Sracek, O. Mechanisms of arsenic enrichment in geothermal and petroleum reservoirs fluids in Mexico. Water. Res. 2010, 44, 5605–5617.
- Welch, A.H.; Lico, M.S.; Hughes, J.L. Arsenic in Ground Water of the Western United States. Groundwater 1988, 26, 333–347.
- Nordstrom, D.K. Worldwide Occurrences of Arsenic in Ground Water. Science 2002, 296, 2143–2145.
- Villaescusa, I.; Bollinger, J.-C. Arsenic in drinking water: Sources, occurrence and health effects (a review). Rev. Environ. Sci. Bio. 2008, 7, 307–323.
- Peters, S.C.; Blum, J.D.; Klaue, B.; Karagas, M.R. Arsenic Occurrence in New Hampshire Drinking Water. Environ. Sci. Technol. 1999, 33, 1328–1333.
- Ali, W.; Rasool, A.; Junaid, M.; Zhang, H. A comprehensive review on current status, mechanism, and possible sources of arsenic contamination in groundwater: A global perspective with prominence of Pakistan scenario. Environ. Geochem. Health 2019, 41, 737–760.
- Wilson, F.H.; Hawkins, D.B. Arsenic in streams, stream sediments, and ground water, fairbanks area, alaska. Environ. Geol. 1978, 2, 195–202.
- Webster, J.G.; Nordstrom, D.K.; Smith, K.S. Transport and Natural Attenuation of Cu, Zn, As, and Fe in the Acid Mine Drainage of Leviathan and Bryant Creeks. In Environmental Geochemistry of Sulfide Oxidation; ACS Symposium Series; ACS: Washington, DC, USA, 1993; Volume 550, pp. 244–260.
- Li, X.; Zhu, X.; Qi, X.; Li, K.; Wei, Y.; Wang, H.; Hu, J.; Hui, X.; Zhang, X. Pyrolysis of arsenic-bearing gypsum sludge being substituted for calcium flux in smelting process. J. Anal. Appl. Pyrolysis 2018, 130, 19–28.
- Liu, X.; Xu, H.; Wang, L.; Qu, Z.; Yan, N. Surface nano-traps of Fe0/COFs for arsenic(III) depth removal from wastewater in non-ferrous smelting industry. Chem. Eng. J. 2020, 381, 122559.
- Krysiak, A.; Karczewska, A. Arsenic Extractability in Soils in The Areas of Former Arsenic Mining and Smelting, SW Poland. Sci. Total Environ. 2007, 379, 190–200.
- Matschullat, J. Arsenic in the geosphere—A review. Sci. Total Environ. 2000, 249, 297–312.
- Peshut, P.J.; Morrison, R.J.; Brooks, B.A. Arsenic speciation in marine fish and shellfish from American Samoa. Chemosphere 2008, 71, 484–492.
- Chilvers, D.C.P.P.J. J. Global cycling of arsenic. In Lead, Mercury, Cadmium and Arsenic in the Environment; Wiley: New York, NY, USA, 1987; pp. 279–301.
- Anastasia, F.B.; Kender, W.J. The Influence of Soil Arsenic on the Growth of Lowbush Blueberry. J. Environ. Qual. 1973, 2, 335–337.
- Goodroad, L.L.; Caldwell, A.C. Effects of Phosphorus Fertilizer and Lime on the As, Cr, Pb, and V Content of Soils and Plants. J. Environ. Qual. 1979, 8, 493–496.
- Levander, O.A. Metabolic interrelationships between arsenic and selenium. Environ. Health Perspect. 1997, 19, 159–164.
- Bobrowicz, P.; Wysocki, R.; Owsianik, G.; Goffeau, A.; Ułaszewski, S. Isolation of Three Contiguous Genes, ACR1, ACR2 and ACR3, Involved in Resistance to Arsenic Compounds in the Yeast Saccharomyces cerevisiae. Yeast 1997, 13, 819–828.
- Bednar, A.J.; Garbarino, J.R.; Ferrer, I.; Rutherford, D.W.; Wershaw, R.L.; Ranville, J.F.; Wildeman, T.R. Photodegradation of roxarsone in poultry litter leachates. Sci. Total. Environ. 2003, 302, 237–245.
- Rutherford, D.W.; Bednar, A.J.; Garbarino, J.R.; Needham, R.; Staver, K.W.; Wershaw, R.L. Environmental fate of roxarsone in poultry litter-Part II: Mobility of arsenic in soils amended with poultry litter. Environ. Sci. Technol. 2003, 37, 1515–1520.
More
