You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Nur Zaida Zahari.
Heavy metal pollution in the environment is a major concern for humans as it is non-biodegradable and can have a lot of effects on the environment, humans as well as plants. At present, a solution to this problem is suggested in terms of a new, innovative and eco-friendly technology known as phytoremediation. Bast fiber plants are typically non-edible crops that have a short life cycle. It is one of the significant crops that has attracted interest for many industrial uses because of its constant fiber supply and ease of maintenance. Due to its low maintenance requirements with minimum economic investment, bast fiber plants have been widely used in phytoremediation.
- phytoremediation
- bast fiber plants
- heavy metals
- hemp
1. Morphology and Characteristics of Bast Fiber Plants (Hemp, Kenaf, Jute and Flax)
Bast fibre is a natural fibre derived from the bast environment of certain dicotyledonous angiosperm plant stems. It is made up of cellulose and hemicellulose combined with a lignin or pectin mixture. In this preseaperrch, the potential of four different fiber plants from various places in the uptake of heavy metals from contaminated soil was highlighted. The four fiber plants are Hemp (Cannabis sativa), Kenaf (Hibiscus cannabinus), Jute (Corchorus olitorius) and Flax (Linum usitatissimum) (Table 1).
Table 1.
Morphology and specifics characteristics of bast fiber plants (Hemp, Kenaf, Jute and Flax).
| Fiber Plants | Morphology | |||||
|---|---|---|---|---|---|---|
| Roots | Stems | Leaves | Flowers | Seeds | Reference | |
| Hemp ( | Cannabis sativa | ) | Root system is well developed with depth of about 1 to 1.5 m | The stems are normally hollow with diameter ranging from 5 to 25 mm. The base and top stem have different diameters. Mature plant reaches up to 5 m | ||
Hemp is a member of the Cannabaceae plant family, and the fibre derived from this plant is one of the strongest forms of natural fibre [10][5]. It has the potential to be an environmentally friendly and a highly sustainable crop if it is well managed. On the other hand, Kenaf and Jute come from the same family of Malvacea. Kenaf is a non-wood fiber that can be used for reinforcement and it is the world’s third traditional crop after wood and bamboo, which originate in Asia and Africa [11][6]. Jute fibers are totally biodegradable as it is partially wood [12][7]. Flax is a member of the Linaceae family of plants, and because its exceptional qualities, Flax fibres are significant raw materials for textiles [12][7]. Flax and Hemp do not have much difference because they are both cellulose fibers, except that Hemp has ten chromosomes (2n = 20), whereas Flax has 15 pairs of chromosomes (2n = 30) [13][8]. Kenaf and Jute are woody-stemmed herbaceous dicotyledons grown in the tropics and subtropics.
2. Application of Bast Fiber Plants (Hemp, Kenaf, Jute and Flax)
Fiber plants are useful not only for phytoremediation but also in a variety of other fields in the world (Table 2). The bast fibre of hemp plants is used in the automotive industry and textile industry, whereas the whole plant part is used for feedstock and biofuel. Hurds are used for paper production and as a building material such as fiberglass. Hemp oil from the seeds is used in shampoos, soaps and bathing gels. The seeds are also applicable in the food industry as hemp milk and are used as a salad dressing. Technical commercial products such as oil paints, ink and coatings are also produced by these plants [18][9]. However, the usage of the plants is based on the quality of the hemp. On the other hand, Jute is the second most important fiber plant in the world, and it is also one of the cheapest-grown fiber plants in the tropical region. It is traditionally used to manufacture packaging materials such as sacking, ropes, twines and carpet-backing cloth. Moreover, diversified Jute is also used in the production of home textiles, composites, geotextiles, paper pulp, technical textiles, chemical products, handicrafts and fashion accessories. The woody central core is used as a rural building material for fences, fuel and for charcoal-making. In the Philippines, the leaves of Jute are used to treat headaches [19][10].
Table 2.
World countries ranking of producing fibre plants.
| Types of Fiber Plants |
Hemp ( | Cannabis sativa | ) | Kenaf ( | Hibiscus cannabinus | ) | −1 | ) | Jute ( | Corchorus olitorius | ) | Flax ( | Linum usitatissimum | ) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Ranking | |||||||||||||||||
| Roots | Leaves | |||||||||||||||||
| The first true leaves are single leaflets; later leaves become palmate compounds. The second leaf pair consists of three leaflets per leaf, the third leaf pair has five leaflets per leaf, and so on, up to eleven leaflets per leaf | Male flowers and female flowers available. Female flowers are more compact | Hemp seeds are achenes seeds. Seeds are ellipsoid in shape, 2 to 7 mm long and 2 to 4 mm wide in diameter. Seeds vary in colour from light brown to dark green | [ | |||||||||||||||
| Shoots | ||||||||||||||||||
| 1 | China | India | India | Russia | ||||||||||||||
| Hemp ( | Cannabis sativa | ) | Pb | 14 | 38.2 | 16.5 | ] | 23.5[1] | ||||||||||
| [ | 33 | ] | [ | 19 | ] | Kenaf ( | Hibiscus cannabinus | ) | It has a prolific root system with a long taproot and extensive lateral roots | |||||||||
| 2 | It mainly has unbranched stems and grows up to 4.5 m tall | Young leaves are simple and entire. Divided leaf can produce 3 to 10 entire young leaves prior to the first divided leaf | It produces large showy, light yellow, creamy coloured flowers that are bell-shaped and widely | open. The flowers are solitary, short-stalked and auxiliary and are 8 to 13 cm in diameter with 5 petals, 5 sepals and numerous stamens |
The seeds are normally brown with 6 mm long and 4 mm wide. The seeds of Kenaf are produced by the fruits, known as fruit capsules in 1.9 to 2.5 cm long and 1.3 and 1.9 cm in diameter with many seeds, around 20 to 26 | [15] | [2] | |||||||||||
| Canada | China | Bangladesh | Canada | |||||||||||||||
| Pb | 14.6 | 2.22 | 2.07 | [36] | [20] | Jute ( | Corchorus olitorius | ) | It has an extensive lateral branching and deep tap root system | The height range of the Jute plant is between 2 and 4 m. The stems are about 1 to 2 cm in diameter with few branches. The colour of the stem, petiole and leaf varies. | The leaves are edible with a bitter taste. Leaves are usually 6–10 cm long and 3.5–5 cm broad | |||||||
| Cd | 2.82 | It consists of small pale-yellow flower, bracts lanceolate, 2 to 3 cm wide, sepals 3 mm long and petals are 5 mm long | Seeds are greyish- black and angled | [ | ||||||||||||||
| 3 | United States of America | 16 | Thailand | China | ] | 0.23Kazakhstan[3] | ||||||||||||
| 0.37 | [ | Flax ( | Linum usitatissimum | ) | It has short and branched tap root that can extend to a depth, of1 m, with side branches spreading to 30 cm | |||||||||||||
| 36 | ] | [ | 20 | ] | 4 | FranceIt has one main stem, but two or more branches (tillers) may develop from the base when plant density is low or with high soil nitrogen levels | The leaves are normally small and lance- shaped | The flowers parts are normally in units of five and can range from a dark to a very light blue, white or pale pink | The seeds are flat, oval and pointed at one end. Normally the seeds are covered in mucilage, giving it a high shine | [17] | [4] | |||||||
| Brazil | ||||||||||||
| Uzbekistan | ||||||||||||
| China | ||||||||||||
| Cd | 1.03 | 0.55 | 0.98 | [33] | [19] | 5 | Chile | Vietnam | Nepal | |||
| Zn | United States | |||||||||||
| 688.6 | 323.1 | 156 | [ | 36] | [20] | 6 | North Korea | Cuba | South Sudan | India | ||
| Zn | 66.8 | 40.0 | 54.5 | [33] | [19] | 7 | Indonesia | Zimbabwe | ||||
| Kenaf ( | Hibiscus cannabinus | ) | Pb | 2.43 | - | 8.9 | [ | 8 | Pakistan | Egypt | ||
| 9 | Pakistan | Vietnam | ||||||||||
| 10 | Cambodia | Bhutan | ||||||||||
| References | [22] | [11] | [23] | [12] | [24] | [13] | [25] | [14] |
Kenaf also has its own uses and one of them is paper production. Kenaf paper is stronger and more resistant to yellowing compared wood paper and it requires fewer bleaching agents. Furthermore, Kenaf seeds produce edible oil, which is one of the best cooking oils. Dried Kenaf leaves are consumed as a vegetable in some countries because they contain 30% crude protein. The fruit of Kenaf helps in lowering blood pressure and the presence of vitamin C and antioxidants in Kenaf help in fighting some diseases. Kenaf will be used in new applications such as medicines, textiles, natural fiber compounds and environmental cleaning [20][15]. Flax is used for fruit, medications and textiles and has therefore been used for food processing. It has been of considerable significance for human civilization and growth for more than 8000 years. For many years, Flax was commonly used for the manufacture of fabrics, although nowadays, oil is the main source in production [21][16].
3. Case Study on Phytoremediation of Heavy Metals Pb, Zn and Cd by Bast Fiber Plants
In this restudyearch, Hemp (Cannabis sativa), Kenaf (Hibiscus cannabinus), Jute (Corchorus olitorius) and Flax (Linum usitatissimum) were chosen to compare their potential for phytoremediation of Pb, Cd and Zn in the soil (Table 3). Hemp plants were harvested from agricultural activities with acidic soil value. The concentrations of these metals were higher in the root than in the leaves and shoots. Hemp can tolerate high concentrations of Zn and most of the Zn absorbed is retained in the roots [26][17]. The uptakes of these heavy metals are significantly influenced by the pH of the soil. This statement is supported by the study caried out by Gray et al. [27][18], where the results showed that increasing the pH will cause a significant reduction in the concentration of cadmium in clover, lettuce, carrot and ryegrass.
Table 3. Heavy metal concentration in Bast Fiber Plants. Listed tissues represent those with the highest concentration of metals in the roots, leaves and shoots.
| Types of Fiber Plants |
Metals | Concentration (mg/kg | ||||||
|---|---|---|---|---|---|---|---|---|
| 28 | ||||||||
| ] | ||||||||
| [ | ||||||||
| 21 | ||||||||
| ] | ||||||||
| Pb | 329.66 | - | 867.55 | [37] | [22] | |||
| Cd | 0.87 | - | 0.36 | [8] | [23] | |||
| Cd | 0.25 | - | 0.14 | [38] | [24] | |||
| Zn | 233.0 | - | 264.0 | [30] | [25] | |||
| Zn | 114 | 65 | - | [39] | [26] | |||
| Zn | 377.78 | 133.33 | - | [40] | [27] | |||
| Jute ( | Corchorus olitorius | ) | Pb | 21.74 | - | - | [41] | [28] |
| Pb | 367.83 | 370.43 | - | [31] | [29] | |||
| Cd | 163 | - | 48 | [31] | [29] | |||
| Cd | 261.83 | 41.35 | - | [42] | [30] | |||
| Zn | 148.53 | 151.42 | - | [42] | [30] | |||
| Flax ( | Linum usitatissimum | ) | Pb | 104.4 | 14.5 | 30.2 | [33] | [19] |
| Pb | 310.56 | - | - | [34] | [31] | |||
| Cd | 13.06 | - | - | [34] | [31] | |||
| Cd | 8.69 | 1.62 | 7.27 | [33] | [19] | |||
| Zn | 255.71 | - | - | [34] | [31] | |||
| Zn | 211.8 | 32.6 | 62.9 | [33] | [19] | |||
Research conducted by Nizam et al. [28][21], highlighted that the concentration and uptake of Pb by the shoot were significantly higher than the root in the Kenaf plant. Most of the varieties grown in Pb contaminated soil accumulated more Pb in shoots than roots, indicating that Pb was easily transported from root to shoot in Pb-contaminated soil. This could be related to the Pb content and its relationship with other essential ions during nutrient uptake. Other studies by Shehata et al. [8][23] mention that Kenaf plants were irrigated with wastewater, and sulfur soil addiction with humic acid was used as foliar spraying and it showed the significant highest accumulation of cadmium, which was 0.87 mg/kg in the roots and 0.36 mg/kg in the shoots. They noticed that humic acids are the most active components in soil and compost as it improves the uptake and accumulation of heavy metals in the tissues’ plant [29][32]. Cecília et al. [30][25], studied the phytoremediation of zinc and the results showed that Kenaf is able to absorb 233 mg/kg of zinc in the roots and 264 mg/kg in the shoots.
Furthermore, the studies about phytoremediation in untreated industrial wastewater from textile factories by Ahmed and Slima [31][29] show that there was very high concentration of Cd in the roots with 261.83 mg/kg and 41.35 mg/kg in the shoots of the Jute. In contrast, the concentration of Pb in the roots was 367.83 mg/kg, whereas in the shoots it was 370.43 mg/kg. This finding shows that the nutrients in the roots and shoots were decreased significantly because of contamination stress. Lead (Pb) is a toxic heavy metal that can inhibit plant growth, seedling development and root elongation [32][33]. They also state that Flax is a fibre plant that is suitable for growing in industrially polluted areas because its root system removes significant amounts of heavy metals from the soil and can be used as a potential crop for cleaning the soil of heavy metals [33][19]. Hosman et al. [34][31], studied the bioremediation potential of Flax under different concentration of Pb, Cd and Zn. The average ability of the Flax plant to remove heavy metals from soil was 49% for Cd, 68.6% for Pb and 71.76% for Zn. Following that, the highest accumulation of Cd was found in the root, whereas the highest accumulation of Pb and Zn was found in the capsule. He also reported that by increasing the metal concentration in the soil, there was a gradual increase in metal uptake in the Flax plant. Several phytotoxicity effects were observed when these metals exceeded the endogenous level [35][34].
4. Enhancing Phytoremediation of Heavy Metals of Bast Fiber Plants by Chemical and Microbiological Amendments
The phytoremediation potential of bast fiber plants can be increased by using chemical amendments in the soil and microbial enhancement through inoculation in the roots of plants. Chemical amendments play a key role in compensating for the relatively low heavy metal availability in soil, and it helps the plants’ uptake and translocates metals toward the shoot [43][35]. Previous studies have reported that various chelators are employed to increase the solubility of metals in soil, including 1,2-cyclohexane-diaminetetraacetic acid (CDTA), ethylene glycol tetraacetic acid (EGTA) and diethylene-triaminepentaacetic acid (DTPA) [44,45,46][36][37][38]. One of the most effective chelating agents is ethylenediaminetetraacetic acid (EDTA), which can increase the solubility, absorption and complexation of metals (including Pb ions in soil) [5,47,48,49][39][40][41][42]. Furthermore, metal-EDTA complexes may form and function to significantly boost Pb ion absorption by plant roots and translocate them to shoots [50][43]. Hasan et al. [51][44] reported that metallothioneins produced by certain genes could withstand conditions where metal stress is present in the environment. Furthermore, this metal-binding protein with low molecular weight can facilitate the metal ion into the plant cells and translocate them via the xylem. In phytoremediation technologies, the addition of nutrients to plants may results in healthy plant growth with the development of flowers, leaves and branching of the root system, and can thus increase the level of uptake contaminant in the study area. However, an excessive amount of nutrients given to the plants can result in a significant reduction in plant growth. This symptom is known as nutrient toxicity. In a nutrient-enriched environment, the bioavailable fraction of metals may be reduced because of the binding to the nutrient anions. The uptake of heavy metals in plants may also be affected by competition since nutrient cations compete with the metal for uptake sites [52][45]. Thus, the uptake of the metal under investigation decreases with an increasing concentration of nutrients. However, a generous availability of nutrients promotes plant growth, which in turn creates an increasing number of uptake sites for metal in plants. This may increase the uptake as well as the metal concentrations in plants.
Interactions between plants and microbes are crucial factors in determining the efficiency of phytoremediation [53][46]. These interactions are implicated to play an essential role in plant metal uptake. The beneficial microbes associated with plants directly improve the efficiency of the phytoremediation process by altering metal accumulation in plant tissues and indirectly by promoting shoot and root biomass production. Whiting et al. [54][47], reported that the biomass and zinc concentration in the shoots of Thlaspi caerulescens has been increased with the presence of rhizospheric bacteria. These bacteria can promote plant growth by inhabiting the plant roots [55][48] and are known as plant growth-promoting rhizobacteria (PGPR) [56][49]. The generation of phytohormones, specialized enzymatic activity, nitrogen fixation in the atmosphere and pathogen-depressing chemicals such sidephores and chelating compounds all contribute to the role of PGPR in promoting plant growth [57][50]. Sidephores and chelating compounds have been shown to promote plant growth even in the presence of heavy metals [58][51]. 1- aminocyclopropane- carboxylic acid deaminase is another plant growth-promoting compound that has been studied in relation to heavy metals (ACC deaminase). ACC is an intermediate of ethylene produced by stressed plants, and bacteria that produce ACC deaminase can reduce ethylene levels in plants, promoting plant growth [59][52].
In another study, Belimov et al. [60][53] discovered that bacteria containing ACC deaminase can improve plant growth in metals-polluted conditions. Meanwhile, Braud et al. [61][54], studied the phytoextraction of agricultural Cr and Pb with sidephore- producing bacteria, and highlighted that the inoculated Maize plant with bacteria enhanced the bioavailability and uptake of Cr and Pb. Khan et al. [62][55], investigated the (Ni) accumulation of mycorrhizal and non-mycorrhizal Flax plants at various concentrations of Ni, i.e., 0, 250, 350 and 500 ppm. He reported that the accumulation of metals was higher in mycorrhizal than in non-mycorrhizal plants. Additionally, mycorrhizal plants showed noticeably greater growth and development than non-mycorrhizal plants. The production of phytohormones by Arbuscular Mycorrhizal Fungi (AMF) can improve nutrient and water uptake as well as improve metal bioavailability and aid in the phytoremediation process [63][56]. Figure 21 shows the mechanism of plant-microbe association that supports metal phytoremediation.
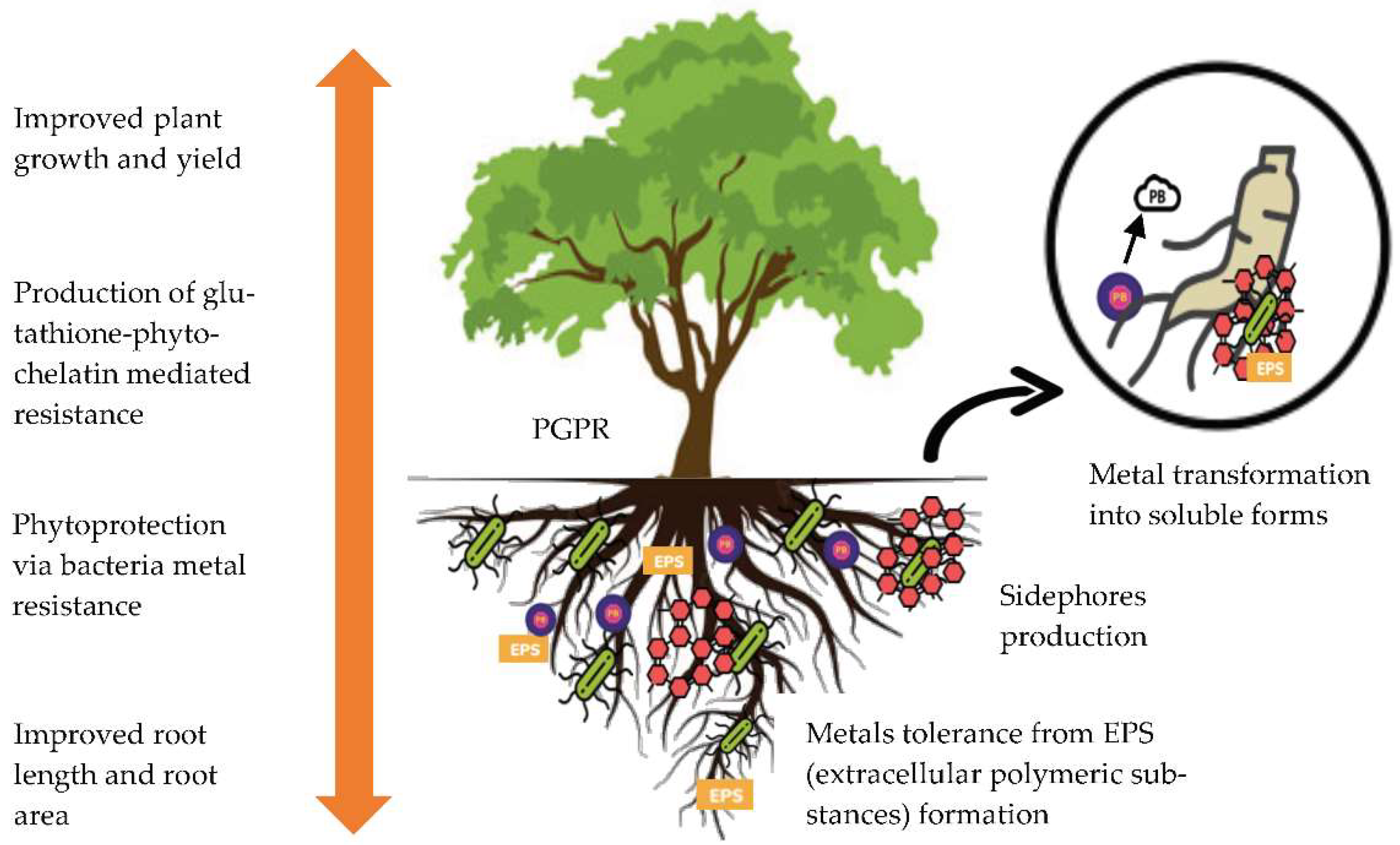
Figure 21.
The mechanism of plant-microbe association that supports metals phytoremediation.
5. Molecular Mechanisms Involved in Microbial Resistance to Heavy Metals
Microorganisms have been involved in the mechanisms of adapting to heavy metals either in water or soil [64][57]. Some metals, such as copper, nickel and cobalt, are given to microorganisms as micronutrients for use in redox processes, to stabilise molecules through electrostatic interactions, to act as components of various enzymes and to regulate osmatic pressure. Otherwise, non-essential metals are recognized as having little nutritional value and may be toxic to microorganisms. To overcome the toxicity value, there are six metal mechanisms that exist in the microorganism, including the exclusion of the permeability barrier, intra- and extra-cellular sequestration, active transport efflux pumps, enzymatic detoxification and reduction in the sensitivity of cellular targets to metal ions.
References
- Ramesh, M. Hemp, jute, banana, kenaf, ramie, sisal fibers. In Handbook of Properties of Textile and Technical Fibres; Woodhead Publishing: Cambridge, UK, 2018; pp. 301–325.
- Mohd, H.A.B.; Arifin, A.; Nasima, J.; Hazandy, A.H.; Khalil, A. Journey of kenaf in Malaysia: A Review. Sci. Res. Essays 2014, 9, 458–470.
- Roy, S.; Lutfar, L.B. Bast fibres: Jute. In Handbook of Natural Fibres, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2020; Volume 1, pp. 39–59.
- Kozłowski, R.M.; Mackiewicz-Talarczyk, M. Introduction to natural textile fibres. In Handbook of Natural Fibres, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2020; Volume 1, pp. 1–13.
- Rupasinghe, H.; Davis, A.; Kumar, S.; Murray, B.; Zheljazkov, V. Industrial Hemp (Cannabis sativa subsp. sativa) as an Emerging Source for Value-Added Functional Food Ingredients and Nutraceuticals. Molecules 2020, 25, 4078.
- Tholibon, D.; Tharazi, I.; Sulong, A.B.; Muhammad, N.; Ismail, N.F.; Radzi, M.K.F.M.; Radzuan, N.A.M.; Hui, D. Kenaf Fiber Composites: A Review on Synthetic and Biodegradable Polymer Matrix (Komposit Gentian Kenaf: Satu Ulasan bagi Sintetik dan Biodegradasi Polimer Matrik). J. Kejuruter. 2019, 31, 65–76.
- Chand, N.; Fahim, M. Natural Fibers and Their Composites. In Tribology Natural Fiber Polymer Composites; Woodhead Publishing: Cambridge, UK, 2008; 58p.
- Divashuk, M.G.; Alexandrov, O.S.; Razumova, O.v.; Kirov, I.v.; Karlov, G.I. Molecular Cytogenetic Characterization of the Dioecious Cannabis sativa with an XY Chromosome Sex Determination System. PLoS ONE 2014, 9, e85118.
- Schluttenhofer, C.; Yuan, L. Challenges towards Revitalizing Hemp: A Multifaceted Crop. Trends Plant Sci. 2017, 22, 917–929.
- Kumari, N.; Choudhary, S.B.; Sharma, H.K.; Singh, B.K.; Kumar, A.A. Health-promoting properties of Corchorus leaves: A review. J. Herb. Med. 2018, 15, 100240.
- Cruz, J.C.; House, L.A.; Blare, T.D. Global Overview of Hemp Production and the Market of Hemp-Derived CBD in the U.S. Available online: https://agris.fao.org/agris-search/search.do?recordID=US2022245382 (accessed on 28 October 2022).
- Alexopoulou, E.; Papatheohari, Y.; Christou, M.; Monti, A. Origin, Description, Importance, and Cultivation Area of Kenaf. Green Energy Technol. 2013, 117, 1–15.
- FAO. World Food and Agriculture—Statistical Yearbook 2021; FAO: Rome, Italy, 2021.
- Saleem, M.H.; Ali, S.; Hussain, S.; Kamran, M.; Chattha, M.S.; Ahmad, S.; Aqeel, M.; Rizwan, M.; Aljarba, N.H.; Alkahtani, S.; et al. Flax (Linum usitatissimum L.): A Potential Candidate for Phytoremediation? Biological and Economical Points of View. Plants 2020, 9, 496.
- Nurazzi, N.M.; Shazleen, S.; Aisyah, H.A.; Asyraf, M.; Sabaruddin, F.; Mohidem, N.; Norrrahim, M.N.F.; Kamarudin, S.; Ilyas, R.A.; Ishak, M.; et al. Effect of silane treatments on mechanical performance of kenaf fibre reinforced polymer composites: A review. Funct. Compos. Struct. 2021, 3, 045003.
- Ludvíková, M.; Griga, M. Transgenic flax/linseed (Linum usitatissimum L.)—Expectations and reality. Czech J. Genet. Plant Breed. 2015, 51, 123–141.
- Placido, D.F.; Lee, C.C. Potential of Industrial Hemp for Phytoremediation of Heavy Metals. Plants 2022, 11, 595.
- Gray, C.W.; McLaren, R.G.; Roberts, A.H.C.; Condron, L.M. Effect of soil pH on cadmium phytoavailability in some New Zealand soils. N. Z. J. Crop Hortic. Sci. 1999, 27, 169–179.
- Angelova, V.; Ivanova, R.; Delibaltova, V.; Ivanov, K. Bio-accumulation and distribution of heavy metals in fibre crops (flax, cotton and hemp). Ind. Crops Prod. 2004, 19, 197–205.
- Ćaćić, M.; Perčin, A.; Zgorelec, Ž.; Kisić, I. Evaluation of heavy metals accumulation potential of hemp (Cannabis sativa L.). J. Cent. Eur. Agric. 2019, 20, 700–711.
- Agric, K.J.E.; Nizam, M.U.; Wahid-U-Zzaman, M.; Rahman, M.M.; Kim, J.-E. Phytoremediation Potential of Kenaf (Hibiscus cannabinus L.), Mesta (Hibiscus sabdariffa L.), and Jute (Corchorus capsularis L.) in Arsenic-contaminated Soil. Korean J. Environ. Agric. 2015, 35, 111–120.
- Uddin, M.N.; Wahid-Uz-Zaman, M.; Rahman, M.M.; Islam, M.S.; Islam, M.S. Phytoremediation Potentiality of Lead from Contaminated Soils by Fibrous Crop Varieties. Am. J. Appl. Sci. Res. 2016, 2, 22–28.
- Shehata, S.M.; Badawy, R.K.; Aboulsoud, Y.I.E. Phytoremediation of some heavy metals in contaminated soil. Bull. Natl. Res. Cent. 2019, 43, 189.
- Bada, B.S. Bioremediation of textile effluent polluted soil using kenaf (Hibiscus cannabinus Linn.) and composted market waste. J. Appl. Sci. Environ. Manag. 2015, 19, 773–776. Available online: https://www.ajol.info/index.php/jasem/article/view/131249 (accessed on 28 October 2022).
- Santos, G.C.G.d.; Rodella, A.A.; de Abreu, C.A.; Coscione, A.R. Vegetable species for phytoextraction of boron, copper, lead, manganese and zinc from contaminated soil. Sci. Agric. 2010, 67, 713–719.
- Arbaoui, S.; Evlard, A.; Mhamdi, M.E.W.; Campanella, B.; Paul, R.; Bettaieb, T. Potential of kenaf (Hibiscus cannabinus L.) and corn (Zea mays L.) for phytoremediation of dredging sludge contaminated by trace metals. Biodegradation 2013, 24, 563–567.
- Jaafar, M.N. Determination of Zinc Uptake by Kenaf (Hibiscus cannabinus L.) for Phytoremediation. Bachelor’s Thesis, Universiti Teknologi Petronas, Seri Iskandar, Malaysia, 2011. Available online: https://utpedia.utp.edu.my/8927/ (accessed on 28 October 2022).
- Osundiya, M.O.; Ayejuyo, O.O.; Olowu, R.A.; Bamgboye, O.A.; Ogunlola, A.O. Bioaccumulation of heavy metals in frequently consumed leafy vegetable grown along Nigeria-Benin Seme Border, West Africa. Adv. Appl. Sci. Res. 2014, 5, 1–7. Available online: https://www.cabi.org/ISC/abstract/20143131014 (accessed on 28 October 2022).
- Ahmed, D.A.; Slima, D.F. Heavy metal accumulation by Corchorus olitorius L. irrigated with wastewater. Environ. Sci. Pollut. Res. 2018, 25, 14996–15005.
- Hassan, M.S.; Dagari, M.S.; Babayo, A.U. Effect of Citric Acid on Cadmium Ion Uptake and Stress Response of Hydroponically Grown Jute Mallow (Corchorus olitorius). J. Environ. Anal. Toxicol. 2016, 6, 375.
- Hosman, M.E.; El-Feky, S.S.; Elshahawy, M.I.; Shaker, E.M. Mechanism of Phytoremediation Potential of Flax (Linum usitatissimum L.) to Pb, Cd and Zn. Asian J. Plant Sci. Res. 2017, 7, 30–40. Available online: https://www.researchgate.net/publication/319666532_Mechanism_of_Phytoremediation_Potential_of_Flax_Linum_usitatissimum_L_to_Pb_Cd_and_Zn (accessed on 28 October 2022).
- Vargas, C.; Pérez-Esteban, J.; Escolástico, C.; Masaguer, A.; Moliner, A. Phytoremediation of Cu and Zn by vetiver grass in mine soils amended with humic acids. Environ. Sci. Pollut. Res. 2016, 23, 13521–13530.
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead uptake, toxicity, and detoxification in plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136.
- Zulfiqar, U.; Jiang, W.; Xiukang, W.; Hussain, S.; Ahmad, M.; Maqsood, M.F.; Ali, N.; Ishfaq, M.; Kaleem, M.; Haider, F.U.; et al. Cadmium Phytotoxicity, Tolerance, and Advanced Remediation Approaches in Agricultural Soils; A Comprehensive Review. Front. Plant Sci. 2022, 13, 773815.
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003.
- Marques, A.P.G.C.; Rangel, A.O.S.S.; Castro, P.M.L. Remediation of Heavy Metal Contaminated Soils: Phytoremediation as a Potentially Promising Clean-Up Technology. Crit. Rev. Environ. Sci. Technol. 2009, 39, 622–654.
- Gielen, H.; Remans, T.; Vangronsveld, J.; Cuypers, A. Toxicity responses of Cu and Cd: The involvement of miRNAs and the transcription factor SPL7. BMC Plant Biol. 2016, 16, 145.
- Doncheva, S.; Moustakas, M.; Ananieva, K.; Chavdarova, M.; Gesheva, E.; Vassilevska, R.; Mateev, P. Plant response to lead in the presence or absence EDTA in two sunflower genotypes (cultivated H. annuus cv. 1114 and interspecific line H. annuus × H. argophyllus). Environ. Sci. Pollut. Res. 2013, 20, 823–833.
- Najeeb, U.; Ahmad, W.; Zia, M.H.; Zaffar, M.; Zhou, W. Enhancing the lead phytostabilization in wetland plant Juncus effusus L. through somaclonal manipulation and EDTA enrichment. Arab. J. Chem. 2017, 10, S3310–S3317.
- Tompsett, S.L.; Smith, D.C. Mercury in Biological Materials. J. Clin. Pathol. 1959, 12, 219.
- Sun, B.; Zhao, F.J.; Lombi, E.; McGrath, S.P. Leaching of heavy metals from contaminated soils using EDTA. Environ. Pollut. 2001, 113, 111–120.
- Hosseini, S.S.; Lakzian, A.; Halajnia, A.; Razavi, B.S. Optimization of EDTA and citric acid for risk assessment in the remediation of lead contaminated soil. Rhizosphere 2021, 17, 100277.
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.W. Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. J. Hazard. Mater. 2011, 185, 549–574.
- Hasan, M.K.; Cheng, Y.; Kanwar, M.K.; Chu, X.Y.; Ahammed, G.J.; Qi, Z.Y. Responses of plant proteins to heavy metal stress—A review. Front. Plant Sci. 2017, 8, 1492.
- Greger, M. Metal Availability, Uptake, Transport and Accumulation in Plants. In Heavy Metal Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2004; pp. 1–27.
- Rane, N.R.; Tapase, S.; Kanojia, A.; Watharkar, A.; Salama, E.-S.; Jang, M.; Yadav, K.K.; Amin, M.A.; Cabral-Pinto, M.M.; Jadhav, J.P.; et al. Molecular insights into plant–microbe interactions for sustainable remediation of contaminated environment. Bioresour. Technol. 2021, 344, 126246.
- Whiting, S.N.; de Souza, M.P.; Terry, N. Rhizosphere bacteria mobilize Zn for hyperaccumulation by Thlaspi caerulescens. Environ. Sci. Technol. 2001, 35, 3144–3150.
- Kloepper, J.W.; Schroth, M.N. Plant growth-promoting rhizobacteria on radishes. In Proceedings of the IV International Conference on Plant Pathogenic Bacteria, Angers, France, 27 August–2 September 1978; Available online: https://www.researchgate.net/publication/284682983_Plant_growth-promoting_rhizobacteria_on_radishes_IV_international_conference_on_plant_pathogenic_bacteria (accessed on 28 October 2022).
- Glick, B.R. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 1995, 41, 109–117.
- Dardanelli, M.S.; Manyani, H.; González-Barroso, S.; Rodríguez-Carvajal, M.A.; Gil-Serrano, A.M.; Espuny, M.R.; López-Baena, F.J.; Bellogín, R.A.; Megías, M.; Ollero, F.J. Effect of the presence of the plant growth promoting rhizobacterium (PGPR) Chryseobacterium balustinum Aur9 and salt stress in the pattern of flavonoids exuded by soybean roots. Plant Soil 2010, 328, 483–493.
- Ahmed, E.; Holmström, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196.
- Glick, B.R.; Todorovic, B.; Czarny, J.; Cheng, Z.; Duan, J.; McConkey, B. Promotion of plant growth by bacterial ACC deaminase. CRC Crit. Rev. Plant Sci. 2007, 26, 227–242.
- Belimov, A.A.; Dodd, I.C.; Hontzeas, N.; Theobald, J.C.; Safronova, V.I.; Davies, W.J. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol. 2008, 181, 413–423.
- Braud, A.; Jézéquel, K.; Bazot, S.; Lebeau, T. Enhanced phytoextraction of an agricultural Cr- and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere 2009, 74, 280–286.
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Ni, F.; Rizwan, M.; Fahad, S.; Hu, L. Morpho-physiological and biochemical responses of tolerant and sensitive rapeseed cultivars to drought stress during early seedling growth stage. Acta Physiol. Plant. 2019, 41, 25.
- Vamerali, T.; Bandiera, M.; Mosca, G. Field crops for phytoremediation of metal-contaminated land. A review. Environ. Chem. Lett. 2009, 8, 1–17.
- Zahari, N.Z.; Tuah, P.M.; Rahim, S.A. Inoculation of Bacillus cereus enhance phytoremediation efficiency of Pistia stratiotes and Eichhornia crassipes in removing heavy metal Pb. IOP Conf. Ser. Earth Environ. Sci. 2021, 847, 012012.
More
