Sargassaceae is the major representative family of Fucales, with
Sargassum-genus representing about 65% of its members. In Europe, native-Sargassaceae species, such as
Cystoseira,
Ericaria, and
Gongolaria, occupy mostly the intertidal rockpools and/or the subtidal region. Particularly,
Cystoseira, accounting for more than 30 species, is one of the most important genera found in the Mediterranean Sea and Atlantic Ocean, essential for biodiversity and ecosystem functioning
[3]. It is also claimed as a promising source of bioactive compounds
[4]. Because of their location, species from this genus are exposed to fluctuations in temperature, seawater salinity, and quantity/quality of light, leading them to adapt and develop protective strategies, such as increasing the content of some pigments to deal with light-irradiance fluctuation
[5].
Sargassum genus, which comprises more than 350 species
[6], is mostly pelagic and is distributed worldwide, despite being found mostly in tropical and subtropical environments
[7]. Lately, the two halopelagic species (
S. fluitans and
S. natans) of this genus have been responsible for
Sargassum tides on the coast of the Caribbean and Western Africa, negatively impacting the marine ecosystems
[7].
Sargassum species have been used for centuries in agriculture as fertilizers, although their applications may go beyond fertilization purposes. Indeed, they have shown particularly interesting properties for the development of plant-biostimulation strategies and/or abiotic-stress mitigation, such as drought
[8][9][8,9]. Moreover, these seaweeds have been explored as raw materials for biofuel production (e.g., methanol and bioethanol), for pharmaceutical, cosmetic, and textile industries, for bioplastic development, and for bioremediation
[10][11][12][10,11,12]. Recently, the species,
Polycladia myrica, widespread throughout the Persian Gulf, northern Australia, Mediterranean Sea, Pacific Ocean, and Indian Ocean tropics and subtropics, was also found to contain phlorotannins with promising UVR-protective effects, granting them great interest for cosmetic applications
[13].
2. Phlorotannins and Main Classes in Fucale
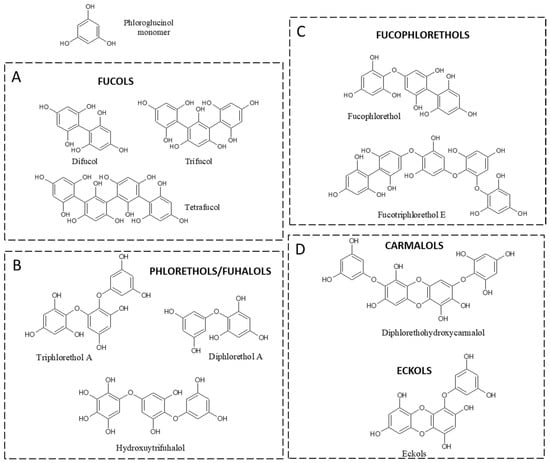
Chemically, PTs represent a class of dehydro-polymers of phloroglucinol (PHG) monomeric units (1,3,5-trihydroxybenzene), with a wide range of molecular weights (126 to 650 KDa), but, most commonly, the molecular weight of these biopolymers ranges from 10 to 100 kDa, divided according to the type of linkage among monomer units: fucols (-C-C- phenyl bonds), fuhalols and phloroethols (-C-O-C- ether bonds differed by their regular sequence of
para- and
ortho-ether bonds, by the presence of additional OH groups), fucophloroethols (both -C-C- and C-O-C- bonds), and eckols/carmalols (containing dibenzodioxin bonds). Eckols differ from carmalols by their usually low molecular weight and by the presence of a phenoxyl substitution (
Figure 1).
Figure 1. Classes of phlorotannins. (A) fucols containing only aryl bonds, (B) phlorethols and fuhalols containing only ether bonds, (C) fucophlorethols containing both aryl and ether bonds, and (D) carmalols and eckols containing dibenzodioxin bonds.
Almost 90% of the total amount of PTs is found in a free state in physodes, that is, cell-cytoplasm, specialized membrane-bound vesicles, while the remaining are in the cell wall, complexed with alginic acid by covalent ester and hemiacetal bonds, acting as a structural component for osmotic-pressure regulation
[30]. They are recognized to accumulate up to 25% of seaweeds’ dry weight (DW), although their levels are highly dependent on the algae species and other factors, including geographic region of growth, age, tissue type, water salinity, season, amount of nutrients, light intensity, and water temperature
[31][32][33][31,32,33]. For example, among the seven species of Fucale, namely
Pelvetia canaliculata,
Fucus spiralis,
Fucus serratus,
Bifurcaria bifurcata,
Himanthalia elongata,
A. nodosum, and
F. vesiculosus, the last two, known to develop mixed belts in the mid-tide zone, are shown to contain the highest content of phenolics (approximately 5.80% DW), while lower levels are found in species that grow in the lower-intertidal level (4.3% DW) and in the upper level of the intertidal zone (3.9% and 3.4% DW for
F. spiralis and
P. canaliculata, respectively)
[34].
Lopes et al.
[35] reported PT variations among species from two Fucale families, namely in
F. spiralis (Fucaceae),
Cystoseira nodicaulis,
Cystoseira tamariscifolia,
Cystoseira usneoides, and
Sargassum vulgare (Sargassaceae), concluding that the maximal levels of PTs were found in the first one, representing about 12 times those found in
S. vulgare. Intermediate and highly variable amounts were also detected among the three
Cystoseira species.
The influence of geographical location and water salinity (positive correlation) on the content of PTs in
F. vesiculosus from the Artic region was clearly shown by Obluchinskaya et al.
[36], who registered levels between 72.4 and 158.1 mg PHG equivalents/g DW algae, depending on sampling locations. Moreover, Pedersen
[37] reported that the phenolic content of
A. nodosum and
F. vesiculosus increased with increasing salinity in their habitats. Further research confirmed that the decrease in salinity matched with high exudation of
A. nodosum and
F. vesiculosus phenolics in the surrounding water, resulting in a significant reduction of the phenolic content of these two species
[38].
Seasonal variation of PTs in Fucale has been screened in distinct species. When following the concentrations of PTs in five perennial-Sargassacean species from the coast of the Sea of Japan, Kamiya et al.
[39] registered large variations throughout the year, but a relatively similar fluctuation pattern among the five species, consisting of a maximum in summer, followed by a decrease towards winter and an increase in April, was observed. In fact, although some contradictory results have been reported in the literature for Fucale species, such as
A. nodusum and
Fucus, most of the data suggest that the production of PTs is maximum during the summer, matching the period of the greatest sun-exposure period, and thus, agreeing with the UV-protective functions invoked for these compounds
[34][40][41][42][34,40,41,42]. Interestingly, some authors have also reported higher production of PTs in seaweeds growing at higher latitudes, where water temperatures tend to be lower, rather than in lower latitudes, where water temperatures are more temperate. Indeed, the species,
Durvillaea antarctica, from South-East Pacific, was found to contain considerably greater phlorotannin levels when collected in higher latitudes (closer to the South Pole) than those collected in mid and lower latitudes (closer to the equator)
[43]. In part, the fact that this species is more adapted to subantarctic regions and strong wave forces, could explained such observations, since these can be an important factors for stimulating the synthesis of structural-function PTs rather than UV-protective ones, although this was not assessed by the authors.
Noteworthy, the aforementioned variables accumulate with changes in the degree of phlorotannin polymerization, which is also greatly influenced by algae species and biotic and abiotic restrictions, further increasing the structural diversity of PTs and making their elucidation difficult. In fact, most published data only reported total PTs levels among macroalgae samples, rather than elucidating differences in their profiles. In the case of Fucales, the analysis of the composition of PTs was restricted mainly to the families, Fucaceae and Sargassaceae, particularly, the genera
Fucus,
Ascophyllum, and
Cystoseira [44][45][46][47][44,45,46,47].
In Fucaceae,
F. vesiculosus is by far the most-studied species in relation to PTs. Among others, the UHPLC-DAD-ESI-MS
n analysis, carried out by
our
esearchers' group on the ethyl acetate fraction of a hydroacetonic extract obtained from this algae species, revealed the presence of common fucols, fucophlorethols, fuhalols, together with several other PTs derivatives of varying degrees of polymerization, ranging from 3 to 22 phloroglucinol units. Moreover, possible new PTs, including fucofurodiphlorethol, fucofurotriphlorethol, and fucofuropentaphlorethol, have tentatively been identified in this species
[48].
Notably, when analyzing PTs-enriched fractions of aqueous and hydroethanolic extracts of three macroalgae by UPLC-MS, Tierney and coworkers
[45] suggested that
A. nodosum and
P. canaliculata contained predominantly larger PTs (degree of polymerization (DP) of 6–13 monomers), compared to
F. spiralis (DP of 4–6 monomers). The complexity of PTs constituents was also referred to for Sargassaceae, particularly the
Sargassum genus. In agreement with other work focusing on the
Sargassum species, Li et al.
[49] reported the predominance of fuhalol-type phlorotannins in a PTs-rich fraction of
S. fusiforme (DP 2–10 monomers), and the detection of other relevant compounds, particularly phlorethols and fucophlorethols with varying degree of polymerization (DP 2–11 monomers); they also reported newly discovered eckols and carmalol derivatives. In general, the authors identify many challenges in the structural elucidation of PTs, which hinder the establishment of relationships between the composition of PTs and the bioactivity of the extract and, consequently, the full implementation of these natural resources by the industry. Thus, a concerted effort on the part of the algae community to develop effective and standard methods for the analysis of PTs is crucial.
3. Extraction Processes
Phlorotannins, as tannins in general, have been preferentially extracted using solid-liquid extraction (SLE) with hydroacetonic mixtures, although other solvents have been attempted. Concordantly, when comparing different extraction solvents, Wang et al.
[50] obtained higher efficiency in extracting PTs from
F. vesiculousus with hydroacetone (70%) rather than hydromethanol, hydroethanol, hydroethyl acetate, or water (at 20 °C or 70 °C), with a total of 393 mg PGE/g extract (78.6 mg PGE equivalents/g DW algae), although the highest extraction yield was obtained in water extracts. In turn, to optimize the extraction of PTs from the same species, Catarino et al.
[48] recently tested various extraction conditions and concluded that extraction with 67% acetone (
v/
v), a solvent-solid ratio of 70 mL/g, and a temperature at 25 °C produced the highest extraction yield (28%). This is similar to the previous study, but with a lower content of total phlorotannin (10.7 mg PGE/g extract), highlighting the variability among algae samples. Recently, natural deep eutectic solvents (NADESs) were developed, consisting of mixtures, with different proportions of choline chloride, lactic acid, malic acid, betaine, glucose, and glycerin
[51]. Although it was an SLE extraction, it can be considered an evolution towards greener methods due to the solvent used. Moreover, the authors found that using water solutions of the prepared NADES can improve the extraction yield of PTs from the algae,
F. vesiculosus and
A. nodosum, achieving values similar to those obtained with the most common organic solvents
[51].
More efficient and environmentally friendly extraction procedures, including microwave-assisted extraction (MAE), ultrasonic-assisted extraction (UAE), pressurized solvent extraction (PSE), supercritical fluid extraction (SFE), and enzyme assisted extraction (EAE) have recently emerged as alternative methods to the conventional SLE extractions using organic solvents. All these new techniques were already applied to extract compounds from algae or, at least, to obtain algal extracts enriched in some compounds
[52][53][54][52,53,54]. So, their application to PTs extraction was expected. In fact, recently, Meng et al.
[55] reviewed these techniques’ application to PTs extraction from several brown macroalgae. In general, the included studies aimed to obtain rich extracts to evaluate their biological activities; therefore, the extraction methodology was not the main issue.
Nevertheless, it is interesting to notice that some groups changed their extraction methodology over the years and still obtained extracts rich in PTs. One of those examples is Valentão and co-workers who started using an SLE methodology with a mixture of acetone:water (7:3). Later on, the same group used the UAE technique to obtain PTs-rich extracts from several
Fucus species. Although the authors’ aim was not to compare the two extraction methodologies and the amounts reported in the same cases but in different units, it seems that UAE did not improve the PTs amount in the studied macroalgae
[16][56][16,56]. Another example that can be highlighted is Shikov and co-workers who used their previously prepared NADES to extract PTs from
F. vesiculosus under UAE conditions. However, the extraction yield did not improve, compared to the authors’ previous data
[51]. It is worth mentioning that the extraction time was reduced to 60 min, which is an advantage
[57].
Focusing only on the extraction of PTs, it seems evident that SFE, although very efficient in the extraction of other metabolites, has not been extensively used in the extraction of PTs
[55]. EAE seems to be an efficient methodology, but there are only a few reports
[58], and its cost may be an obstacle to its large-scale use.
Few macroalgae species have been subjected to different techniques, making it difficult to establish a proper comparison because the amount of PTs extracted depends on the location and collection time
[59][60][59,60]. For example,
F. vesiculosus PTs were extracted using different percentages of ethanol and SLE
[61], UAE
[62], and PSE
[63] techniques. The reported results showed that ethanol percentage influenced the PTs content, but it is also possible to conclude that PSE was the most efficient. A higher amount was extracted (3690 mg gallic acid equivalents/100 g DW seaweed) in just 4.68 min, whereas the other techniques used 30 min and 24 h, respectively, for UAE and SLE, and amounts below 60 mg of gallic acid equivalents/100 g DW seaweed. In addition, MAE was demonstrated to successfully extract PTs in larger amounts and with less extraction time
[31][64][65][31,64,65]. This technique was also applied in
F. vesiculosus [66], however, further studies are needed in Fucales species.
Although the use of non-conventional extraction methods is still scarce, a few considerations may be made regarding their use. MAE, UAE, and PSE require shorter extraction times, and, consequently, lower energy consumption. However, they may cause degradation if high temperatures are reached. They can be suitable for large-scale production, but the cost is relatively high. Naturally, SLE is the more accessible and less expensive technique to use on a large scale; unfortunately, it also involves solvents that are less environmentally friendly.