Micro-sized sensors have become a hot topic in electroanalysis. Because of their excellent analytical features, microelectrodes are well-accepted tools for clinical, pharmaceutical, food safety, and environmental applications. In this brief review, we highlight the state-of-art of electrochemical non-enzymatic microsensors for quantitative detection of ascorbic acid (also known as vitamin C). Ascorbic acid is a naturally occurring water-soluble organic compound with antioxidant properties and its quantitative determination in biological fluids, foods, cosmetics, etc. using electrochemical microsensors is of wide interest. Various electrochemical techniques have been applied to detect ascorbic acid with extremely high sensitivity, selectivity, reproducibility and reliability, and apply to in vivo measurements. The review paper aims to give readers a clear view of advances in areas of electrode modification, successful strategies for signal amplification, and miniaturization techniques used in the electroanalytical devices for ascorbic acid. In conclusion, current challenges related to the microelectrodes design, and future perspectives are outlined.
1. Ascorbic Acid: Biological Role and Commercial Applications
L-Ascorbic acid (AA), also known as vitamin C, is an important target for quantitative sensing and monitoring in the biomedical field, food quality control, pharmaceutical, and cosmetic industries. AA plays an important physiological role in cellular redox metabolism by participating in free radical scavenging and immune system boosting. Antioxidant activity of AA helps to prevent certain diseases such as cancer, hypertension, neurodegenerative diseases, age-related muscular degeneration, cataracts, etc.
[1][2][1,2]. AA is involved in the physiology of the nervous system, including the support and the structure of the neurons, and the processes of differentiation, maturation, and neuronal survival
[3]. AA is a co-factor for at least 15 enzymes involved in the biosynthesis of collagen and L-carnitine, peptide hormone activation, tyrosine metabolism, synthesis of norepinephrine from dopamine, etc.
[4].
As the human body is unable to synthesize AA endogenously, it is important to include adequate AA intake in the diet. AA is also used as a nutritional supplement during deficiency states (weakened body immunity, anemia, scurvy). Oral vitamin C produces tissue and plasma concentrations that the body tightly controls. Approximately 70–90% of vitamin C is absorbed at moderate intakes of 70–180 mg/day. High levels of AA are maintained in cells and tissues, and are highest in brain, pituitary gland, and adrenal glands. Relatively low levels of AA are found in extracellular fluids, such as plasma, and saliva. Side effects are not observed from normal AA intake, due to the fact that it is quickly excreted
[5]. At doses above 1 g/day, absorption falls to less than 50% and absorbed, unmetabolized ascorbic acid is excreted in the urine. After single oral doses of vitamin C greater than 2 g daily, gastrointestinal distress and diarrhea are the most common side effects. However, in individuals with renal dysfunction, increased risk of kidney stones is observed as a serious side effect with vitamin C overdose. Here it should be noted that people most commonly use vitamin C for preventing and treating the common cold although the results are inconsistent and research is still ongoing in this field.
AA is widely used in dermatology and the cosmetic industry as a skin conditioning agent. Numerous clinical studies support the use of topically applied AA for photoprotection, anti-aging, and skin-lightening purposes
[6][7][8][9][6,7,8,9]. The function of AA as an antioxidant and an enzyme cofactor is essential in maintaining skin health and preventing skin aging. Vitamin C is a cofactor of prolyl hydroxylase and lysyl hydroxylase, which are enzymes responsible for the hydroxylation of lysine and proline, a key step of collagen biosynthesis. In addition to stabilizing the collagen molecule by hydroxylation, vitamin C also stimulates collagen mRNA production by fibroblasts
[10].
The topical AA treatment of the epidermal surface increases the synthesis of several specific lipids of the skin, enhancing the protective barrier function
[11][12][11,12]. Vitamin C also provides protection against UV-induced photodamage and participates in the repair of oxidative DNA lesions in skin cells
[13]. A topical vitamin C suppressed UVB-induced cell death, apoptosis, DNA damage, reactive oxygen species (ROS) production, and the inflammatory response by down regulating tumor necrosis factor-α (TNF-α) expression and release
[14].
Vitamin C is a major component of commercially available cosmetics for the treatment of skin pigmentation disorders such as freckles, age spots, and melasma. Such dermatological problems can be caused by various factors such as hormonal imbalance during pregnancy or menopause, thyroid disease, side effects from certain medications, genetic predisposition, etc. Emerging evidence has indicated that Vitamin C has therapeutic effects on facial hyperpigmentation, as it reduces melanin synthesis. AA suppresses the catalytic activity of tyrosinase, the rate-limiting enzyme in melanin biosynthesis
[15]. Although the transdermal absorption efficiency of AA is low and its anti-pigmentary and skin-protective mechanisms still need to be clarified, AA has been used widely as a skin-lightening, anti-aging, and anti-inflammatory agent in commercially available cosmetics designed to protect and rejuvenate photoaged skin.
The strong antioxidant activity of AA and its ability to protect oxidizable constituents, including phenolic and flavor compounds, is the main factor for AA to be frequently used as an additive in the food industry to prevent unwanted changes in color and flavor
[16]. AA is used as color fixative, preservative, and acidity regulator in foods such as meat products, bakery products, canned fruit, canned meat, beer, jam, sweets, and fruit juices. Used as a preservative, AA can reduce the risk of mold and other microbial growth, thereby preventing food spoilage and browning.
As an electron donor, AA serves as one of most important low-molecular-weight antioxidants which contributes to the total antioxidant capacity—an important quality indicator of foods and drinks
[17]. Since natural vitamin C is characterized by low thermal stability and a tendency to oxidize easily, each process using elevated temperature causes loss of this vitamin compared to fresh material. These losses can be from 20 to even 90% depending on the temperature level, the duration of the processing operation, and contact with oxygen
[18]. Because vitamin C is easily destroyed by pressing and heat treatment, manufacturers of juices and fruit/vegetable purees use AA as an additive to either renew or improve the overall nutritional value of the product. Otherwise, the specific flavor of AA enhances the taste of some food products. Thus, fruit juices, jams, and candies often benefit from the tinge of acidity that provides the consumer with the diverse taste of fresh fruits.
Concerning the importance and wide application of AA, the reliable quantitative detection of AA became an important issue not only for the routine chemical analysis, but also for clinical biology, and the pharmaceutical and cosmetic industries. Monitoring AA content should be regarded as an essential and relevant task for evaluating the quality of food products, raw materials, and various pharmaceutical formulations, considering the nutritional value and therapeutic AA properties.
2. Electrochemical Microsensors for Ascorbic Acid Determination
The electroanalytical methods have demonstrated advantages over conventional methods such as titrimetric analysis, spectrophotometry, and liquid chromatography, demonstrating higher sensitivity, exceptional selectivity, remarkable repeatability and accuracy, low power requirements, operational simplicity, and cost effectiveness. More importantly, electrochemical sensor devices can be miniaturized, eventually enabling in vivo and in situ detection of the target analyte in the actual microenvironment
[19]. Recent advances in microfabrication, surface modification, and signal processing have aided the design of highly sensitive and efficient microsensors.
It is well known that under micro (or nano) scale sizes electrodes show unique electroanalytical behavior compared with conventional electrodes. As a consequence of the reduced capacitive charging currents and increased mass transport rates, microelectrodes exhibit excellent signal-to-noise (S/N) characteristics, rapid response, and high-speed measurement. The high mass transfer rates allow electroanalytical measurements to be made at low substrate concentrations. Electrochemical microsensors enable samples to be analyzed in real time at the point of care or through implantation. Such an approach is vital for effective diagnoses, monitoring responses to treatment, and thus providing prognoses for chronic medical conditions
[20]. In the last few years, research has convincingly shown that in vivo electrochemistry is one powerful strategy for probing brain chemistry. In vivo monitoring of the dynamics of the changes in neurochemicals becomes more and more important to unveil and understand brain activity and function
[21].
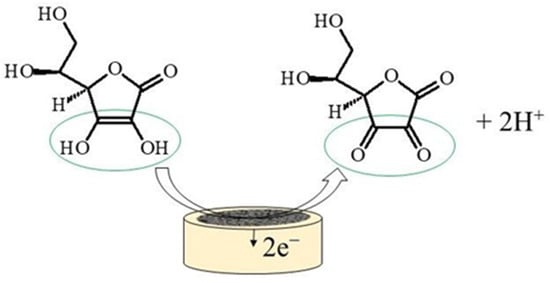
Ascorbic acid is a ketolactone with two ionizable hydroxyl groups. pK
1 is 4.2 and pK
2 is 11.6, thus the ascorbate monoanion is the dominant form at physiological pH, while in acidic medium (pH < 4.2) AA remains in the protonated form. A generalized mechanism of the electrocatalytic oxidation of AA to dehydroascorbic acid, is shown in
Figure 1. The oxidation of AA includes the transfer of two electrons and 2H
+ ions, to produce dehydroascorbic acid, which was proven to be followed by an irreversible solvation reaction at pH lower than 4.0. This irreversible reaction yields an electroinactive product (2,3-diketogulonic acid) easily adsorbable on the electrode surface, which can result in electrode fouling
[17].
Figure 1.
Electrocatalytic oxidation of ascorbic acid.
Chronoamperometry and voltammetric techniques—cyclic voltammetry (CV), differential pulse voltammetry (DPV), and square wave voltammetry (SWV) (Figure 2) can be used as an efficient alternative, providing an affordable and accurate approach for fast quantitative determination of AA. Amperometry is based on the application of a constant potential to a working electrode, and the subsequent measurement of the current generated by the oxidation or reduction of an electroactive analyte. The resulting steady-state current is proportional to the bulk concentration of the analyte. Voltammetry is a potentiodynamic technique, based on measuring the current arising from oxidation or reduction reactions at the electrode surface when a controlled potential variation is imposed.
In the DPV technique, short pulses (10–100 ms) with limited amplitude (1–100 mV) are superimposed on a linear ramp. The current is immediately measured before the pulse application and at the end of the pulse; the difference between the currents is recorded and plotted versus the potential. The procedure effectively reduces the capacitive current, due to the direct current ramp. Accordingly, DPV shows a higher sensitivity and selectivity compared to CV due to the enhanced discrimination of Faradaic currents.
In SWV, a symmetrical square-wave pulse is superponated to a staircase wave. The duration of the pulse is equal to the length of the staircase, and the superponation is obtained in such a way that the forward pulse of the square wave coincides with the first half of that staircase. The first current is measured at the end of the forward square-wave pulse, and the second one is measured at the end of the return square-wave pulse; the signal is obtained as an intensity of the resulting differential current. The change in current between potential steps, is plotted versus the potential. SWV is a fast and powerful technique with extremely low detection limits comparable with those of chromatographic techniques.
The emergence of microsensor devices has provided new directions to various applications including disease diagnosis, pharmacology, and food safety. Depending on the application field (biomedical, pharmaceutical, or food safety), there are specific requirements to the sensor’s operational characteristics. In regard to the linear dynamic range, microsensors are required to detect the analyte in the actual nano-, micro-, or millimolar levels. Limits of detection and quantification also should be adequate to the lowest possible levels of the analyte in the particular sample. In terms of selectivity, the sample matrices (biofluids, tissues, foods, etc.) contain numerous different and specific molecules that have similar behavior to the target analyte and undergo electrochemical conversion. Therefore, the methodology of the selectivity experiments must adequately reflect the intended microsensor mechanism and application.
The next parts of the review present the most recent and innovative works published on microelectrodes and their application for AA detection in biological, pharmaceutical, and food samples, mostly focused on original studies reported from 2015 to date.