Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 5 by Romano Silvestri.
β-Catenin is a key multiplayer protein of the Wingless/integrase-1 (Wnt)/β-catenin signaling pathway. The Wnt-signaling pathway plays key roles in regulating cell fate, proliferation, tissue homeostasis and maintenance and embryogenesis. Aberrant accumulation of β-catenin in the cell nucleus as a result of deregulation of the Wnt/β-catenin pathway is found in various types of cancer.
- β-catenin
- inhibitor
- small molecule
1. Introduction
β-Catenin is a key multiplayer protein of the Wingless/integrase-1 (Wnt)/β-catenin signaling pathway [1][2]. The Wnt-signaling pathway plays key roles in regulating cell fate, proliferation, tissue homeostasis and maintenance and embryogenesis [3][4][5]. Without a Wnt ligand, β-catenin is phosphorylated in the cytosol by the destruction complex. After phosphorylation, β-catenin dissociates from the complex and undergoes ubiquitination by β-transducing repeats-containing proteins (β-TrCP) and then degradation by proteasome. Alternatively, β-catenin undergoes phosphorylation by the destruction complex and then is ubiquitinated by the β-TrCP, the result of which is a minimal amount of β-catenin remaining in the cells [6] (Figure 1, left panel) Upon binding with a Wnt ligand, the interaction with Frizzled (Fzd) receptors and low-density lipoprotein receptor-related proteins 5 and 6 (Lrp5/6) triggers downstream events involving the recruitment of Dishevelled 1 (DVL-1) and Axin, following which β-catenin dephosphorylates and translocates into the nucleus. Accumulation of β-catenin in the nucleus prompts the expression of T-cell factor (Tcf) and the lymphoid enhancer-binding factor (LEF) transcriptional factors. It recruits transcriptional coactivators, such as CREB-binding protein (CBP)/p300 [7]; B-cell lymphoma 9 (BCL9) [8][9] and its paralogue BCL9-like (BCL9L) [10]; and Pygopus (Pygo 1 or Pygo 2) [11], and induces epigenetic modifications [12] (Figure 1, right panel). β-catenin may also bind to E-cadherin in the cytoplasm to recruit actin filaments [13]. The Wnt/β-catenin pathway is found hyperactivated in cancer. The accumulation of β-catenin in the cell nucleus triggers mutations, driving the transcription of oncogenes, such as Jun, Axin-2, c-Myc and Cyclin-D1, resulting in the initiation and progression of various types of carcinoma, including colon cancer, hepatocellular carcinoma, pancreatic cancer, lung cancer and ovarian cancer [14][15].
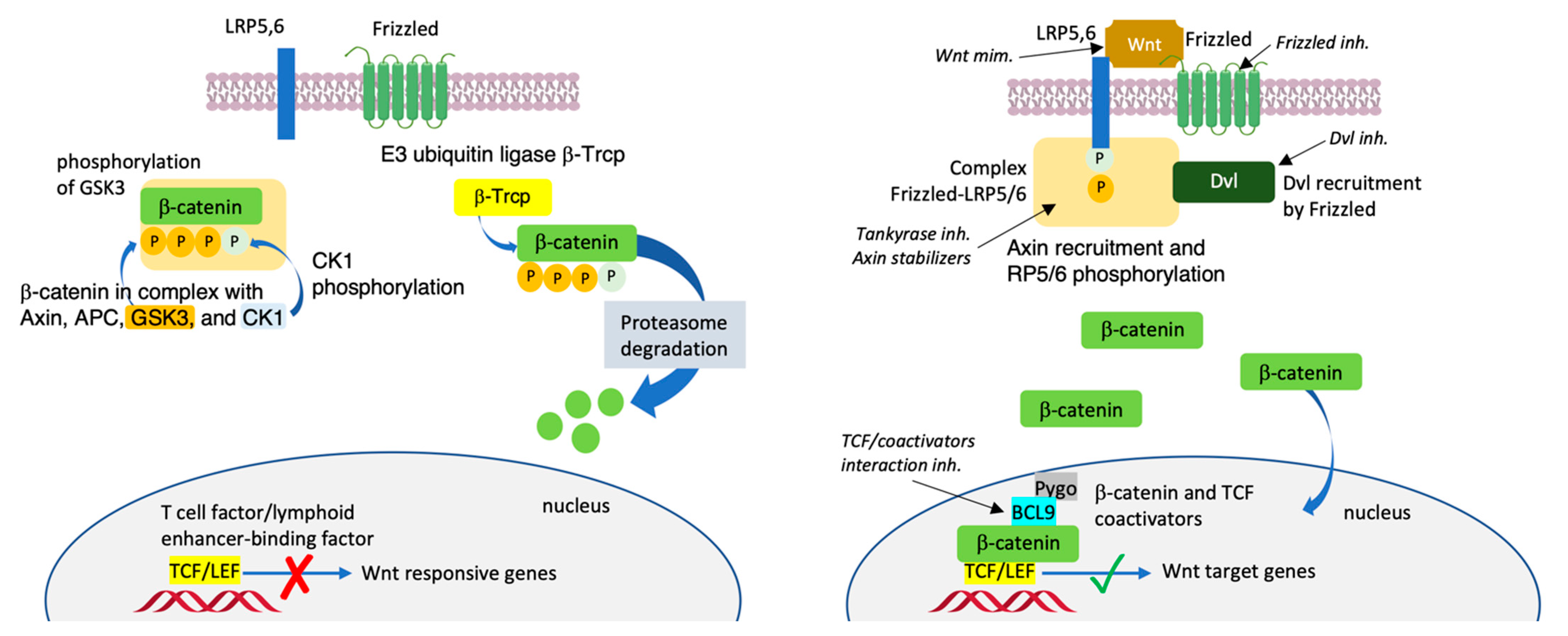
Figure 1. Sketch of the canonical Wnt/β-catenin signaling pathway without a Wnt ligand (left panel) and upon binding with a Wnt ligand (right panel). Molecules being evaluated in clinical studies: Wnt ligand mimetics, inhibitors of Frizzled, Dvl, Tankyrase, TCF/coactivators interactions or Axyn stabilizers.
Various inhibitors of the Wnt/β-catenin pathway have been reported to date. These compounds target the Wnt/β-catenin pathway through different mechanisms: Wnt ligand, its receptor, β-catenin subcellular localization or the β-catenin transcriptional complex. The therapeutic potential of targeting the Wnt/β-catenin pathway has been reported in recent excellent reviews [16][17]. Specific drugs against this signaling pathway for clinical treatments have not been approved so far.
2. Carnosic Acid
De La Roche and co-workers identified some natural compounds, including the carnosic acid (31) from rosemary, that inhibited, in a dose-dependent manner, the binding of β-catenin to BCL9 in vitro, and β-catenin-dependent transcription in CRC cells [18]. Biophysical analysis highlighted the labile α-helix (H1) at the amino terminus of the β-catenin Armadillo repeat domain bordering the BCL9-binding site, as a crucial element for the activity of 31. The mechanism of action of 31 was to promote selectively the proteasomal degradation of unphosphorylated β-catenin in an H1-dependent manner. To identify inhibitors of this interaction, the authors developed an in vitro assay that monitors the binding of His-HD2 (BCL9 homology domain 2) to glutathione S-transferase (GST)-ARD (immobilized on glutathione-coated microplates), using a colorimetric assay to quantify bound His-HD2 after addition of compounds. NMR saturation transfer difference (STD) spectroscopy was used to investigate the interaction of 31 with its target domain using the minimal HD2-binding domain within its N-terminus (four repeats: R4) [19]. Evidence highlighted that the effects of 31 on R4 could explain its in vivo effects on β-catenin. The authors proposed that the metastable H1 also predisposed β-catenin to low-grade aggregation in vivo, and that this is exacerbated by 31, which could earmark β-catenin for proteasomal degradation. Saturation transfer difference (STD) NMR spectroscopy assays were performed to indentify the binding domain of 31 with purified R4 or BCL9 homology domain 2 (HD2). R4 tested positive in this ligand-observed binding assay whereas HD2 was negative. Titration with varying concentrations of R4 allowed researchers to estimate a Kd in the 5–20 μM range. The STD assays unequivocally identify R4 as the molecular target of 31.
3. Bispyrrodinylium-Carboxamide
Hoggard and co-workers designed and synthesized novel small-molecule inhibitors that selectively disrupted β-catenin/BCL9 over β-catenin/cadherin PPIs [20]. The binding mode of new inhibitors was characterized by site-directed mutagenesis and SAR studies. The new inhibitors suppressed the transactivation of canonical Wnt signaling, downregulated the expression of Wnt target genes, and inhibited the growth of Wnt/β-catenin-dependent cancer cells. The authors considered two hot regions at the β-catenin/BCL9 interface: in hot region one, residues D162, E163 and D164 of human β-catenin interact with residues H358 and R359 of human BCL9; in hot region two, residues L366, I369, and L373 of BCL9 interact with a hydrophobic pocket bordering with residues L159, V167, L160, A171 and M174. Compound 32 was provided with two positively-charged pyrrolidino groups and a substituted phenyl ring were introduced to improve the selectivity between α-helix-mediated PPIs. AlphaScreen and ITC studies consistently showed that 32 bound wild-type β-catenin but not BCL9. Compound 32 inhibited the β-catenin/BCL9 PPI, but not β-catenin/E-cadherin PPI, in colorectal SW480, HCT116, HT29 CRC cells and triple-negative breast cancer cells MDA-MB-231 and MDA-MB-436, all overexpressing the Wnt signaling with IC50 values in the low micromolar range. In the AutoDock predicted the binding conformation within β-catenin (PDB 2GL7); main contacts were the H-bonds of the protonated pyrrolidino groups with D145 and E155, the interactions of the mono and difluoro substituted phenyl rings with L178 and L159, respectively, and the contact of the oxygen atom linked to one pyrrolidino group with L156.
4. hsBCL9CT-24
Feng’s group designed peptides based SAH-BCL9B, a previously reported peptide of the BCL9 homology domain 2 (BCL9-HD2) with promising inhibition of the Wnt pathway [21]. To improve activity, the authors focused on stapled peptides and conducted lead optimization studies using full-length BCL9-HD2. Among the peptides synthesized at AnaSpec, hsBCL9CT-24 (33) showed the most potent in vitro activity and exhibited stronger binding affinity to β-catenin compared to BCL9-HD2A. In the homogeneous time resolved fluorescence (HTRF) binding assay, hsBCL9CT-24 yielded a Kd value of 4.21 nM, compared to Kd value of 192.3 nM of SAH-BCL9B. In a modified AlphaScreen that allows greater surface area of β-catenin/BCL9 binding [22][23] to determine the potency of inhibitors in disrupting the β-catenin/BCL9 interaction, hsBCL9CT-24 showed a low Kd value of 4.73 nM. hsBCL9CT peptides were found to be highly permeable and readily taken up by treated cells. hsBCL9CT-24 was superior to SAH-BCL9B as inhibitor of β-catenin transcriptional activity in HCT116 and Colo320DM (dependent on β-catenin and BCL9) cell lines. hsBCL9CT-24 effectively reduced tumor growth in the Colo320DM xenograft and in PDX models of human CRC, showed a favorable pharmacological profile and minimal toxicity. Docking of 33 into the β-catenin hydrophobic pocket (PDB 3SL9) performed by GlideXP Maestro Schrodinger highlighted binding interactions with Ala187, Asp145, Leu178, Met174, Lys170 and Val 167 amino acids in the β-catenin hydrophobic pocket (Figure 2).
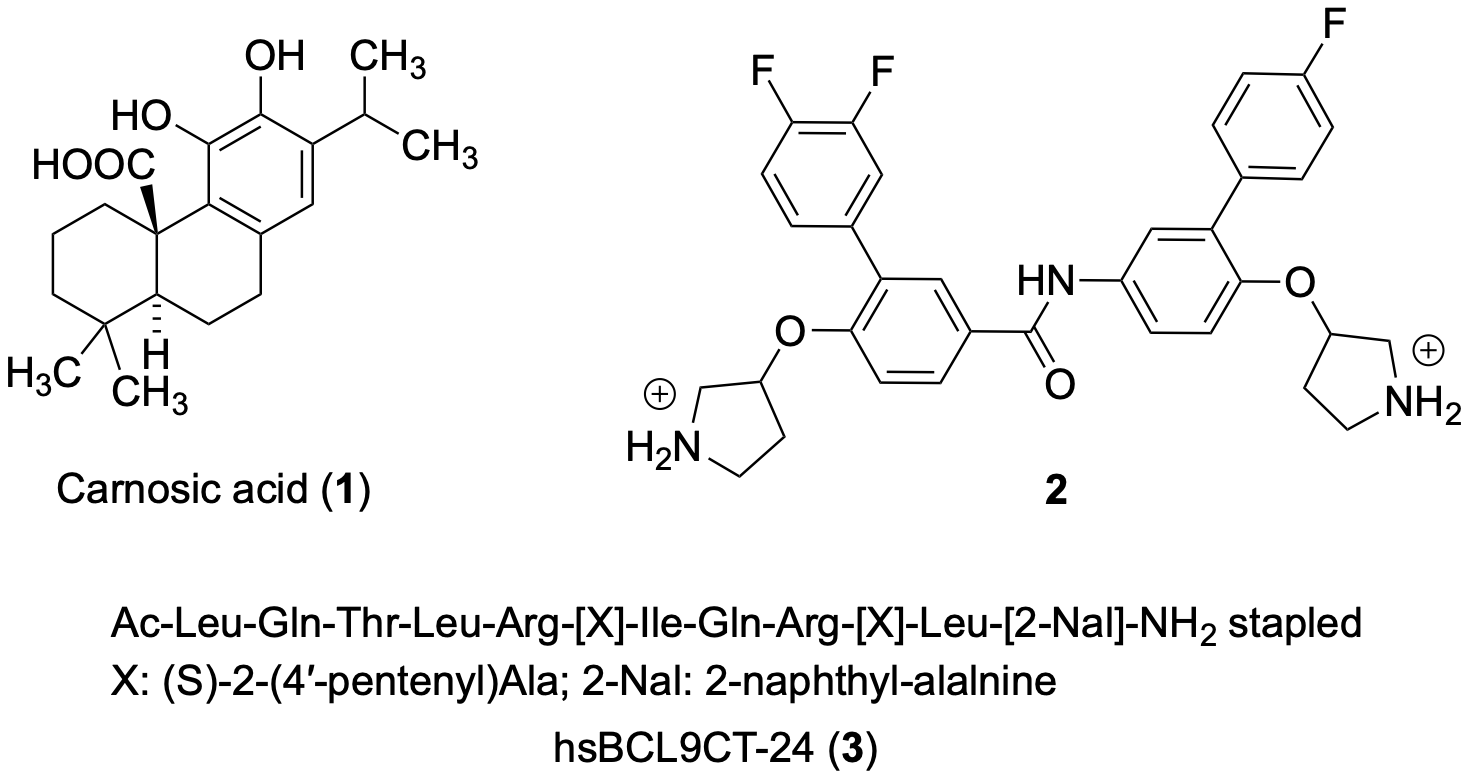
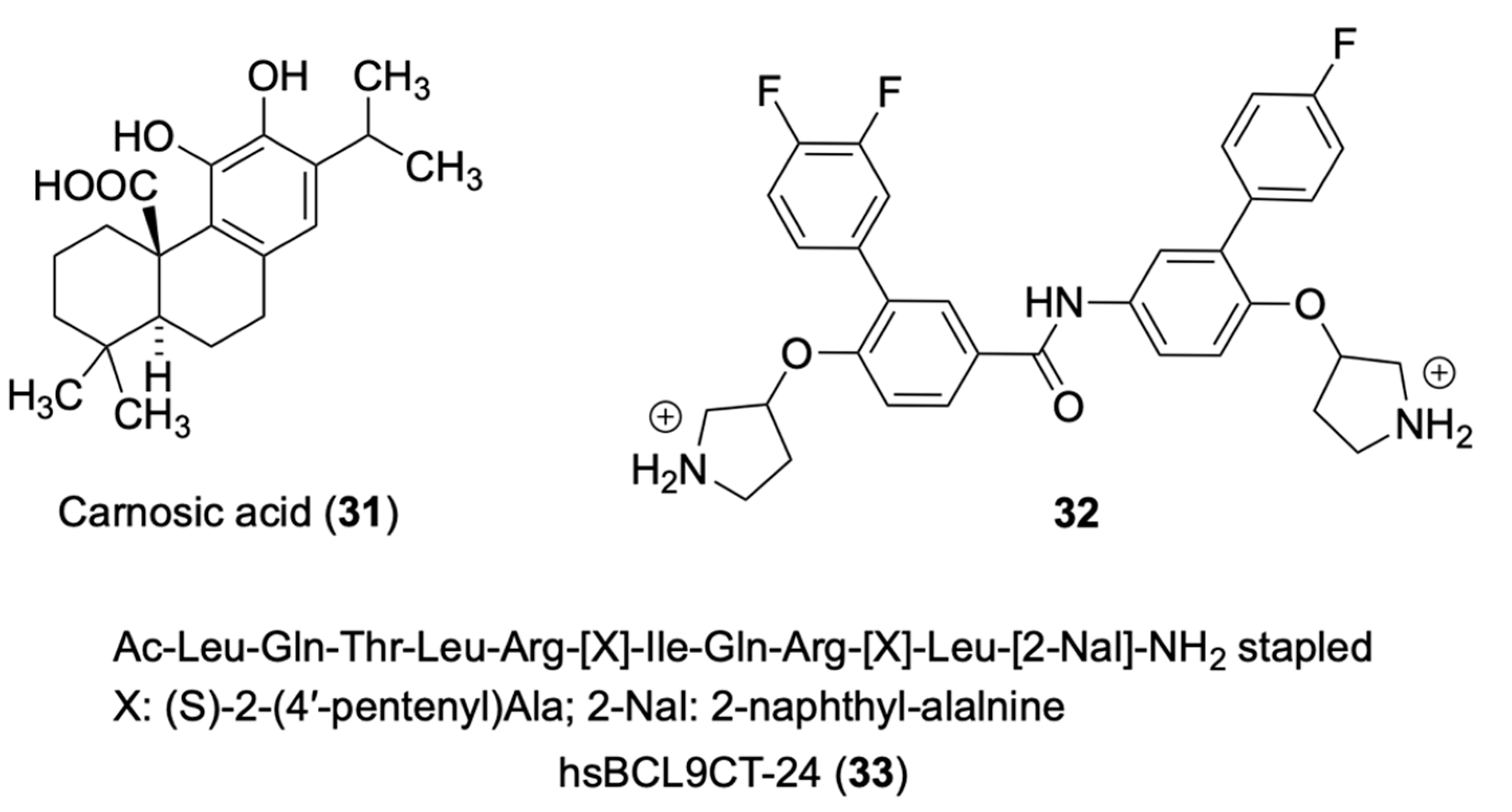
Figure 2. Chemical structures of compounds 31–33 inhibitors of β-catenin to BCL9.
References
- Valenta, T.; Hausmann, G.; Basler, K. The many faces and functions of ß-catenin. EMBO J. 2012, 31, 2714–2736.
- Saito-Diaz, K.; Chen, T.W.; Wang, X.; Thorne, C.A.; Wallace, H.A.; Page-McCaw, A.; Lee, E. The way Wnt works: Components and mechanism. Growth Factors 2013, 31, 1–31.
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205.
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012.
- Nusse, R.; Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999.
- Rubinfeld, B.; Albert, I.; Porfiri, E.; Fiol, C.; Munemitsu, S.; Polakis, P. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science 1996, 272, 1023–1026.
- Hecht, A.; Vleminckx, K.; Stemmler, M.P.; van Roy, F.; Kernier, R. The p300/CBP acetyltransferases function as transcriptional coactivators of β-catenin in vertebrates. EMBO J. 2000, 19, 1839–1850.
- Brack, A.S.; Murphy-Seiler, F.; Hanifi, J.; Deka, J.; Eyckerman, S.; Keller, C.; Aguet, M.; Rando, T.A. BCL9 is an essential component of canonical Wnt signaling that mediates the differentiation of myogenic progenitors during muscle regeneration. Dev. Biol. 2009, 335, 93–105.
- Cantù, C.; Zimmerli, D.; Hausmann, G.; Valenta, T.; Moor, A.; Aguet, M.; Basler, K. Pax6-dependent, but β-catenin-independent, function of Bcl9 proteins in mouse lens development. Genes Develop. 2014, 28, 1879–1884.
- Kotolloshi, R.; Gajda, M.; Grimm, M.-O.; Steinbach, D. Wnt/β-catenin signalling and its cofactor BCL9L have an oncogenic effect in bladder cancer cells. Int. J. Mol. Sci. 2022, 23, 5319.
- Schwab, K.R.; Patterson, T.L.; Hartman, H.A.; Song, N.; Lang, R.A.; Lin, X.; Potter, S.S. Pygo1 and Pygo2 roles in Wnt signaling in mammalian kidney development. BMC Biol. 2007, 5, 15.
- Stewart, D.J. Wnt signaling pathway in non-small cell lung cancer. J. Nat. Cancer Inst. 2014, 106, djt356.
- Huber, A.H.; Weis, W.I. The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell 2001, 105, 391–402.
- Shang, S.; Hua, F.; Hu, Z.-W. The regulation of β-catenin activity and function in cancer: Therapeutic opportunities. Oncotarget 2017, 8, 33972–33989.
- Zhang, Y.; Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165.
- Wang, Z.; Li, Z.; Ji, H. Direct targeting of β-catenin in the wnt signaling pathway: Current progress and perspectives. Med. Res. Rev. 2021, 41, 2109–2129.
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Trans. Targ. Ther. 2022, 7, 3.
- De La Roche, M.; Rutherford, T.J.; Gupta, D.; Veprintsev, D.B.; Saxty, B.; Freund, S.M.; Bienz, M. An intrinsically labile α-helix abutting the BCL9- binding site of β-catenin is required for its inhibition by carnosic acid. Nat. Commun. 2012, 3, 680.
- Kramps, T.; Peter, O.; Brunner, E.; Nellen, D.; Froesch, B.; Chatterjee, S.; Murone, M.; Züllig, S.; Basler, K. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear β-catenin-TCF complex. Cell 2002, 109, 47–60.
- Hoggard, L.R.; Zhang, Y.; Zhang, M.; Panic, V.; Wisniewski, J.A.; Ji, H. Rational design of selective small-molecule inhibitors for β-catenin/B-cell lymphoma 9 protein-protein interactions. J. Am. Chem. Soc. 2015, 137, 12249–12260.
- Feng, M.; Jin, J.Q.; Xia, L.; Xiao, T.; Mei, S.; Wang, X.; Huang, X.; Chen, J.; Liu, M.; Chen, C.; et al. Pharmacological inhibition of β-catenin/BCL9 interaction overcomes resistance to immune checkpoint blockades by modulating Tregcells. Sci. Adv. 2019, 5, eaau5240.
- Zhang, M.; Wisniewski, J.A.; Ji, H. AlphaScreen selectivity assay for β-catenin/B-cell lymphoma 9 inhibitors. Anal. Biochem. 2015, 469, 43–53.
- Takada, K.; Zhu, D.; Bird, G.H.; Sukhdeo, K.; Zhao, J.-J.; Mani, M.; Lemieux, M.; Carrasco, D.E.; Ryan, J.; Horst, D.; et al. Targeted disruption of the BCL9/b-catenin complex inhibits oncogenic Wnt signaling. Sci. Transl. Med. 2012, 4, 148ra117.
More
