Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Daniel Mota-Rojas.
Ensuring the welfare of wildlife under human care requires tools to monitor their health and well-being. Infrared thermography is a non-invasive technique for assessing thermal states that measure the radiation emitted from the skin in distinct anatomical areas, known as thermal windows—anatomical regions with abundant capillaries and arteriovenous anastomosis that facilitate heat exchange with the environment.
- infrared thermography
- thermal status
- pain
- thermal window
1. Introduction
Infrared thermography (IRT) is considered a non-invasive, real-time technique used as a complementary diagnostic tool for several physiological and pathological processes in domestic and wildlife species under human care [1,2,3,4,5][1][2][3][4][5]. The control of heat exchange between body surfaces and the external environment plays a crucial role in regulating body temperature during different physiological phases and/or activities throughout the lifetime of homeotherms. Thermoregulatory adjustments can be induced by changes in environmental temperature and numerous physiological factors including age, fasting and food intake, stressful circumstances, and inflammation status, which can cause changes in internal temperature that result in variations in body surface temperature. The evaluation of surface temperature using infrared thermography—a non-invasive technique that captures images of a specific body region at a distance—represents a valuable tool for monitoring the animals’ physiological status, welfare, and stress responses [6,7][6][7]. The reactions of animals under stressful conditions primarily involve activating the sympathetic system and hypothalamic–hypophysis–adrenal axis (HPA) through the release of effector hormones, such as catecholamines, and glucocorticoid production, respectively. One result of this activation is stress-induced hyperthermia, which consists of an increased core body temperature with consequent temperature changes [8].
As a non-invasive tool, remote evaluation is considered advantageous because it minimizes handling or the use of chemical restraints [9] and prevents the development of processes like stress hyperthermia in small rodents and birds [10]. However, the wide diversity of species under human care has distinct anatomical, morphological, and physiological traits. For example, feathers are thermal insulators that maintain temperatures within normal ranges, but their presence limits the use of IRT, which requires zones with bare skin. Therefore, the eye region and legs are the preferred thermal windows in birds for evaluating superficial temperature changes [10]. In the case of animals with sparse hair, such as elephants, rhinoceroses, and hippopotami, or those that do not have long hair, like giraffes, or equids like zebras, IRT can be used in the abdomen, thorax, or ears. In contrast, for carnivores or species with winter coats, the recommendation is to evaluate windows located in the joints, feet (on the caudal side), or facial region (Figure 1) [1,11][1][11].
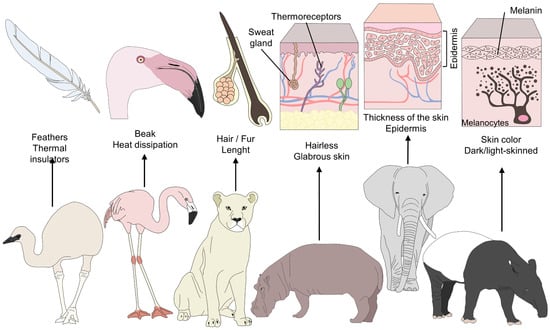
Figure 1. Morphological differences among furred, feathered, and bare-skinned animals. Thermoregulatory strategies in animals depend on various morphological elements. For example feathers serve as a thermal insulator in birds, and beaks facilitate heat dissipation. In contrast, the presence of hair and piloerection form a layer that conserves warm air to prevent any sudden drop in temperature. A thick layer of hair and dark hair color conserve heat and reduce radiation. Finally, skin color and the thickness of glabrous skin influence the amount of heat radiation in different species.
2. Advantages of IRT for Assessing the Thermal Status of Zoo Animals
IRT uses physical laws and surface properties to determine the superficial temperature of animals by measuring the amount of infrared radiation they emit (Figure 2) [12]. One of the main advantages that IRT offers is real-time, non-contact measuring of the surface temperatures of animals from distances of less than 1 m to over 1000 m, depending on species and the specific anatomical region selected [13]. This means that animals do not require extensive handling, physical restraint, or sedation, which is especially important when handling wild animals or those in zoos [1,14,15][1][14][15]. The IRT technique uses a camera with specialized thermal imaging lenses with diamond or germanium optical components and film to capture infrared radiation. A sensor processes this radiation to produce a digital radiometric image converted into a color pattern reflecting the temperatures detected [16].
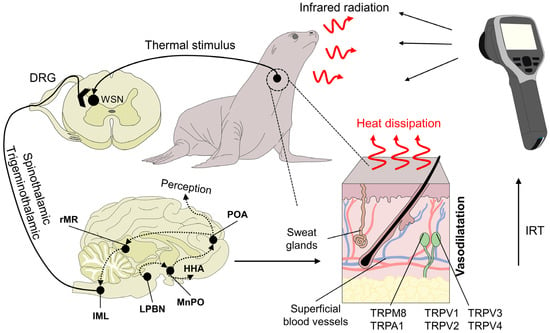
Figure 2. Physiological basis of infrared thermography and mechanisms of heat loss. Peripheral receptors in the dermal tissue, such as transient receptor potential vanilloid (TRPV, TRPV1, TRPV2, TRPV3, and TRPV4) or melastatin-related transient receptors (TRPM8), respond to heat and cold environmental stimuli, respectively. TRP ankyrin (A1) is postulated to have a function in noxious cold sensation and mechanosensation. These receptors initiate the transduction of the stimulus through primary peripheral nerve endings connected to secondary neurons in the spinal cord. Upon reaching this structure, warm-sensitive neurons (WSN) are activated and project the signal along the spinothalamic and trigeminothalamic pathways. Both of these tracts are connected to higher brain structures, such as the lateral parabrachial nucleus (LPBN) and median preoptic nucleus (MnPO) in the preoptic area of the hypothalamus (POA). DRG: dorsal root ganglion. HHA: hypothalamo-hypophyseal-adrenocortical. IRT: Infrared thermography. Their role is to generate behavioral, autonomic, and neuroendocrine responses. One of the autonomic responses to heat stimuli is peripheral vasodilatation by sympatho-adrenergic neurons in the rostral medullary raphe (rMR) and the intermediolateral nucleus of the spinal cord (IML), which increases heat dissipation. This response is captured by the lens of the infrared thermographic camera to generate a radiometric image.
The use of IRT with wild animals has focused primarily on detecting inflammatory processes, but other applications include aspects of thermoregulatory physiology in different taxa, population counts and comparisons, and evaluating the stages of pregnancy. IRT is helpful in the case of pregnancy because increased blood irrigation causes an increase in the surface temperature at the level of the flank in pregnant females. Additionally, it can help determine the association of certain emotional states with temperature changes and indirectly evaluate the habitat or housing of those animals [11,17][11][17].
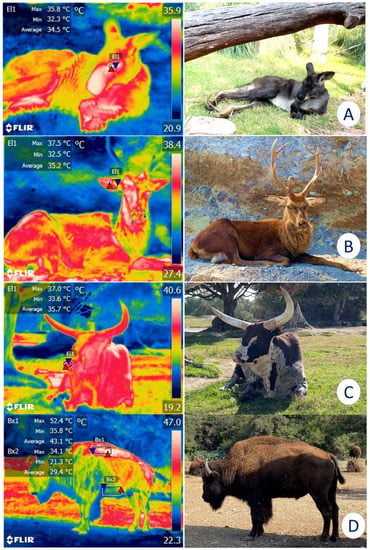
For these reasons, IRT has been proposed as a method for monitoring enclosures and their characteristics to determine whether they comply with legal regulations and satisfy the basic biological needs of the species (e.g., shaded areas, bodies of water, adequate water supply) [18]. For example, alterations in the surface temperatures of elephants have been reported when the micro- or macroclimate of the installations are inappropriate for this species [19]. Similarly, in bears [20] and Bengal tigers (Panthera tigris) [21], IRT has recorded surface cutaneous temperature values and associated them with certain thermoregulatory behaviors, such as piloerection, seeking refuge in shaded areas, or rolling in the dirt to achieve thermoneutrality. This technique has also been used to assess the characteristics of the housing provided, which may favor or inhibit thermal comfort. Figure 3 shows the importance of providing sources of natural shade in enclosures for wildlife under human care since direct solar radiation without a proper resting area can cause heat stress in zoo animals.

Figure 3. Importance of natural or artificial shade for the thermoregulatory behavior of various species. (A). Although this kangaroo (Osphranter rufus) is resting under natural shade, the average temperature of the lacrimal caruncle (El1) is 34.5 °C. (B). A sika deer (Cervus nippon) under shade shows an average temperature of 35.2 °C in the auricular region (El1). (C). The average nasal temperature of 35.7 °C of an ankole-watusi (Bos taurus taurus ankole) under the shade of a tree is depicted. (D). In an American bison (Bison bison), a comparison between the dorsal (Bx1) and ventral (Bx2) regions can be observed. An average temperature difference of 13.7 °C was registered between them. Therefore, shaded areas are a key element for ensuring the thermal comfort of wild animals under human care (Radiometric images and photographs by the reseauthorchers). Maximum temperature (red triangle), and minimum (blue triangle). Thermal images obtained using a FLIR thermal camera.
Concerning thermal physiology, IRT recognizes several thermal windows that can be used for different species, including the African elephant (Loxodonta africana) [22], otters (Lutra lutra and Pteronura brasiliensis) [23], mole rats (Fukomys mechowii and Heliophobius argenteocinereus) [24], ground hornbills (Bucorvidae) [25], and koalas (Phascolarctos cinereus) [26]. Clinically, changes in thermal patterns, such as abnormal or asymmetrical thermal distribution, have been associated with pathologies, alterations in blood circulation, and inflammatory processes. In this area of study, Hilsberg-Merz [27] used IRT to evaluate problems affecting the joints and feet of elephants, rhinoceroses, giraffes, and hippopotami using IRT, while Church et al. [13] diagnosed a respiratory condition in an elephant related to a poorly positioned molar that was affecting the sinus area. In another study, Hurley-Sanders et al. [28] utilized IRT with fluorescein angiography to confirm a compromised vascular system and injury to the right wing in a Chilean flamingo (Phoenicopterus chilensis) that had a clinical history of acute weakness in that limb.
Thermography has also been used for the early diagnosis of diseases such as rabies in raccoons [29] and foot-and-mouth disease in mule deer (Odocoileus hemionus) [30]. Hurley-Sanders et al. [31] used it to monitor the healing process of an injury to the left forelimb of Gregory’s wolf (Canis rufus gregoryi) after a skin graft. Results showed that IRT could aid in the early detection of a poor blood supply to the graft based on the stability of the vascularization of the transplanted skin. Recently, IRT has begun to be used in a field of research where emotional states derived from various stimuli have been found to correlate with thermal responses, especially in primates like rhesus monkeys (Macaca mulatta) [32], gorillas [33] and chimpanzees [34].
However, applying the IRT technique more widely in medical attention for zoo animals and wildlife species under human care requires not only a deep understanding of the anatomy of a wide range of species but also and evaluation of numerous other elements: the fact that humans do not have absolute control over the animal (movement, position relative to the sun, body zones, etc.), and environmental factors (e.g., direct radiation, humidity, rain, wind) that impact the accuracy and interpretation of the temperature readings obtained [1]. Specific body regions have been proposed for using IRT with individual species considering their characteristics and challenges and each one’s morphological properties.
3. Facial Windows
3.1. Ocular Window (Regio Ocularis)
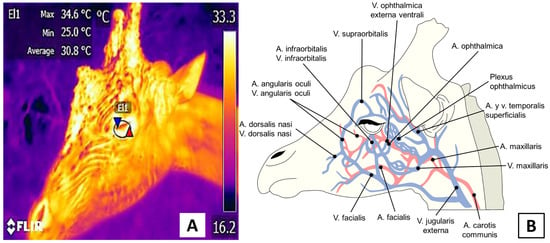
The ocular window assesses circulatory changes and thermal responses in the eye and the proximal area. Its usefulness has been reported in bovines [35], pigs [36], and dogs [37]. This window is surrounded by abundant periocular capillaries branching from the supra and infraorbital arteries. The latter irrigates the lacrimal canaliculi. Because its capillaries have autonomic innervation, they are sensitive to autonomic activation, especially during periods of acute stress. The area of the ocular window and its irrigation are represented in Figure 4.

Figure 4. Depiction of the ocular window of a giraffe (Giraffa camelopardis). (A). A circle, approximately 3 cm in diameter encompasses the periocular region and a small portion of the eyelid. (B). Blood irrigation in this window comes from the supraorbital and infraorbital arteries, whose sympathetic innervation proceeds from branches of the facial nerve.
The lacrimal caruncleis known to have sympathetic-innervated blood vessels that can respond to the activation of the HPA when exposed to a stressor. This is due to the secretion of catecholamines and the vasoconstriction response by its capillaries [38]. This effect has been reported in domesticated species such as sheep [2] and horses [39] during handling and transport, respectively. In those events, the temperature of the lacrimal caruncle has been seen to increase by up to 1 °C. In the case of wildlife species, Narayan et al. [26] evaluated the temperature of the lacrimal caruncle, abdomen, ears, and paws of three koalas born in captivity. They observed that the surface temperature of the ocular region had the greatest consistency and showed a significant increase compared to the ear region (31.6 °C vs. 23 °C, respectively), with a coefficient of variation of 4.83% in contrast to the values obtained from the ear (19.68%). This shows the window’s usefulness for evaluating the body temperatures of animals. Their conclusions are similar to the findings of Melero et al. [40], who compared the thermal windows of five species of marine mammals: two cetaceans, a beluga whale (Delphinapterus leucas) and bottlenose dolphin (Tursiops truncatus), and three pinnipeds, a Patagonian sea lion (Otaria flavescens), a harbor seal (Phoca vitulina), and a Pacific walrus (Odobenus rosmarus divergens). The ocular region was considered appropriate for determining body temperature in the pinnipeds, the ocular region was considered appropriate for determining body temperature. In contrast, in the cetaceans, the internal mucosal region of the open blowhole was suggested as the most stable thermal window for monitoring body temperature non-invasively, as it showed differences of 1 °C compared to the normal rectal temperature of these species.
The studies described above are associated with the clinical usefulness of thermography focused on the ocular region for determining the thermal status of animals, which can be confirmed by similarity with the values obtained by conventional methods, such as rectal temperature. However, the effectiveness of IRT has also been compared to other approaches. Stryker’s [21], for example, compared the thermal response of the eye to standard methods (rectal and axillary readings) and thermoregulatory behaviors in four species of large felines, namely, lions (Panthera leo), jaguars (P. onca), pumas (Puma concolor), and snow leopards (P. uncia). Using IRT, he identified that the surface temperature of the eye was similar to rectal values (39.9 °C and 39 °C, respectively). A second important observation was that the warm-climate felines (lions, jaguars) spent more time lying down (40–84%) than the cold-climate species (pumas, snow leopards) and had relatively less time performing active behaviors (5–10%). That study showed that temperature readings could provide information on the welfare of animals in enclosures when associated with different species’ typical behaviors for adapting to their environment.
A study by South [41] reported a similar response in the European hedgehog (Erinaceus europaeus). There, IRT was applied to monitor hypothermia during hibernation using ocular temperature as a reference in 55 animals (23 males, 32 females). The temperature obtained with an axillary thermometer was significantly lower (1.3 ± 0.18 °C) than that of the periocular region (33.7 °C and 35.5 °C, respectively). Likewise, the ocular reading diagnosed hypothermia more precisely than relying on behavioral and clinical signs, suggesting that IRT is an accurate tool that can aid in evaluating the thermal states of animals and associate them with specific pathological and physiological conditions, thus contributing to the rescue of these species.
In contrast to the reliability and usefulness of IRT described above, a study of 39 Guianan squirrel monkeys (Saimiri sciureus) showed that facial temperature correlated poorly with rectal recordings (r2 = −0.10, 95% IC= −0.27–0.07), as the average difference between the methods was 3.4 °C [42]. These results show that the precision of IRT depends greatly on choosing an adequate region for recording surface temperatures. Similar to descriptions of domesticated species, ocular IRT readings can be used to determine stress levels [2,43][2][43]. A study of giraffes (Giraffa camelopardis) evaluated the thermal response of the ocular and auricular regions under the assumption that, for these animals, feeding could be a stressful event. Results showed a correlation between blood cortisol levels (p = 0.269) and temperature that did not change before or after feeding. A positive relationship was also observed between the eye’s and the ear’s thermal response (Spearman rank p = 0.759, p < 0.0001). The reseauthorchers attributed the absence of any ocular thermal response to a potentially enjoyable emotional state, suggesting that IRT could be useful for identifying emotional states in animals [44]. Vaz et al. [45] found a similar response in African lions (P. leo), where the IRT response at the ocular level was used to understand large felines’ personality and stress physiology, when coordinated with ethological studies and fecal glucocorticoid levels. The reseauthorchers identified three personality types by studying 22 African lions in two locations, (dominant, gentle, and neurotic). The animals had differences in their fecal cortisol metabolite levels, but these did not influence their thermal responses, so it was impossible to correlate body surface temperature with the personality type or age of the animals. One important element is the standardized distance of 1 m between the thermal camera and the ocular region. Another is lens angle, which must be perpendicular (90°) to the sagittal plane to ensure valid readings [46,47][46][47].
As observed in zoo species, unlike in domestic animals, measuring the surface temperature at the ocular level is a remote method to assess body temperature without handling the animals. However, its application to assess the stress degree or ANS activation has yet to be well established. Therefore, as reported in large ruminants, future studies must consider each species’ emotional factor and anatomical differences, as reported in large ruminants [38].
Finally, it is important to consider that the ocular window can be evaluated by other techniques, such as optical coherence tomography (OCT). This method could help evaluate in vivo vascular responses by identifying the reactions of surface ocular blood vessels. Meleppat et al. [48] used this approach with aerial, aquatic, and terrestrial species like the Long Evans rats, gray short-tailed opossums (Monodelphis domestica), white sturgeons (Acipenser transmontanus), and great horned owls (Bubo virginianus). OCT has proven effective in identifying retinal and choroidal vascular structures and retinal layers [49]. Therefore, it can be suggested as a novel tool for diagnosing ophthalmologic diseases since the responses recorded by IRT may differ when animals are exposed to stressors.
3.2. Auricular Window (Regio Auricularis)
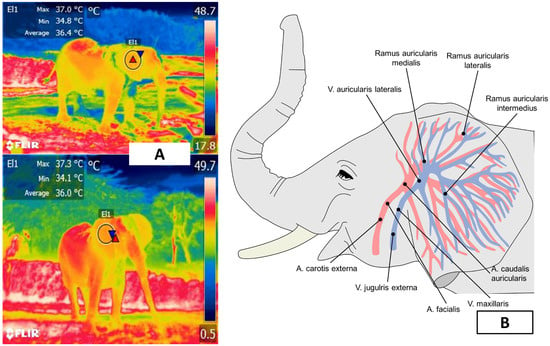
The auricular window is the most important region for thermographic evaluation in elephants. The ears of elephants represent approximately 20% of the total body surface area [50] and have a dense subcutaneous vasculature provided by the auricular arteries and veins (arteria auricularis caudalis) on the caudal surface [51] (Figure 5). The elephants’ ears dissipate as much as 8% of body heat [52], though Hilsberg-Merz [1] mentions that African elephants—the species with the largest ears—can lose up to 30% of excess heat by radiation and convection through the ears. In elephants, the extension of the auricular pavilion compensates for the lack of sweat glands [22].

Figure 5. Auricular window of an African elephant (Loxodonta africana). (A). A circle around the external auditory canal represents the window. (B). The blood supply to the ear comes from the branches of the lateral auricular artery and the medial and marginal areas of the ear.
Because of these characteristics, IRT can assess the auricular region to determine the thermostability of the species and the influence that environmental temperature and/or enclosure design exert on it. Phillips and Heath [50] studied the heat exchange mechanism in four female African elephants (L. africana), finding that surface temperatures of the pinna change in response to environmental temperature through vasomotor action. They further stated that readings proximal to the external auditory meatus were higher than those registered at the extremities of the pinna (39 °C and 18 °C, respectively, at an ambient temperature of 18 °C). Parallel to changes in auricular microcirculation, ear-flapping is another method of thermoregulation that is believed to transfer heat by rotational oscillations [53]. This process involves the rotator, auriculo occipitalis, postauriculatis, platysma myoides, and sphinctor profundus muscles in the Loxodonta genus, and the rotator, auriculo occipitalis, postauricularis, and zygomatico auricularis muscles in the Elephas genus [54]. Therefore, the vasculature and motor control of the ears allow heat dissipation through radiative heat and convection, facilitate heat exchange in that region, and are considered a useful window for evaluating the thermal comfort zone of these species. Ear-flapping dissipates heat by forced convection, thus increasing the rate of heat loss.
A study of six African elephants (L. africana) by Weissenbock et al. [22] reported similar results. They took 325 thermograms of six body regions at times of 5, 10, and 30 m: head, proboscis, torso, forelimb, hindlimb, and ear. The ear lobe and distal areas of the pinna delimited the latter. IRT in this region increased in a range of 33.8–35.3 °C in warm weather after the animals returned to their indoor enclosure. Seventeen minutes after returning, the average ear temperature was 17.1 °C, while after 68 min it increased, revealing areas of maximum and minimum temperatures at 33 °C and 22.2 °C [22]. This area remained the body region with the lowest surface temperature. Intriguingly, the reseauthorchers recorded different thermal patterns in the two ears of the same animal. This difference has also been reported in leporids, where sympathetic innervation can control the temperature of each pinna independently. In contrast to the variability and absence of any established pattern in other thermal windows, auricular IRT associates the thermoregulatory response of the animals with their housing conditions. Therefore, it is essential to (i) provide water for frequent bathing and (ii) consider other morphological characteristics, such as the elephant’s wrinkled skin, which increases the surface area and ensures more efficient heat loss due to water evaporation [50].
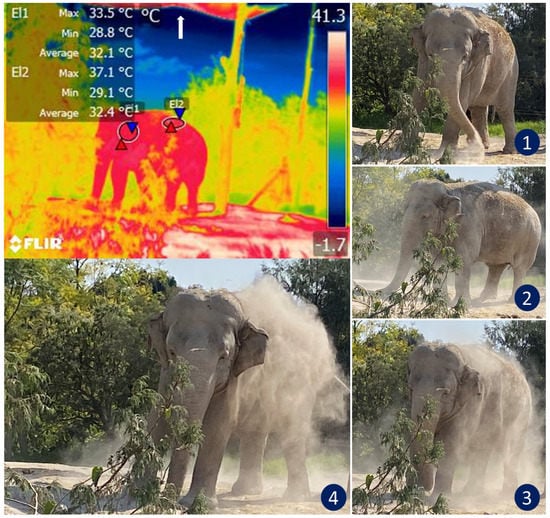
The design of the enclosures also influences thermostability. Here, IRT can help evaluate the thermal responses of animals. Figure 6 illustrates how this thermal window was used to assess the thermal states of African elephants exposed to heat stress as they performed natural thermoregulating behaviors, such as ear-flapping, bathing and splashing in mud. The reseauthorchers of a study of Asian elephants that had recently been moved to a new enclosure observed lethargy in all subjects. Their IRT temperature readings of the ears were similar to those recorded for the body. They considered this an abnormal pattern since the ears of elephants are structures designed for heat dissipation. However, upon analyzing the macro environment of the pachyderms, the authoresearchers found high levels of humidity (95%) and insufficient access to sources of water, two conditions that resulted in heat stress and higher ear temperatures as compensatory mechanisms [19]. In that case, the vapor partial pressure was close to the saturation pressure, thus reducing the evaporation rate and decreasing evaporative heat loss. The only possible response by those animals was to increase the peripheral temperature (increased auricular IRT) to maximize heat loss through radiation and convection. An important point to emphasize in that study is that the entire group showed the same pattern of increased auricular IRT. This made it possible to non-invasively discern between a pathological process in a single individual and a general, simultaneous response by the herd [1].

Figure 6. Thermoregulatory behavior in an African elephant (Loxodonta africana) under heat stress. The sequence of digital images shows dustbathing in an elephant exposed to high temperatures that triggered thermal imbalance (see images 1, 2, 3, and 4). The radiometric image shows the average temperature of the auricular (El1) and dorsal (El2) thermal windows. Under normal conditions, the ear temperature in elephants oscillates between 25 and 31 °C. However, the thermal image shows a temperature in the auricular region as high as 33.5 °C. IRT imaging reveals that although the animal covers its back with dust to thermoregulate, this behavior might be insufficient to achieve thermostability in this environment because the maximum temperature of the dorsum was 37.1 °C. Although the enclosure has a shade mesh as a roof to reduce direct sunlight (white arrow), it is not enough to reduce thermal stress in this species. (Radiometric images and photographs by the reseauthorchers).
IRT has also been utilized to diagnose inflammatory pathologies in Asian elephants (E. maximus). Avni-Magen et al. [55] found that assessing regions like the head, ears, body, and limbs at a distance of 2–3 m identified inflammatory processes with a sensitivity and specificity of 89.2 and 83.4%, respectively, and a negative predictive value (NPPV) of 99.4%. In that study, increases of up to 2° in the delta temperature of the ear surface made it possible to detect inflammatory processes before clinical diagnoses were made due to visible signs. However, due to the low positive predictive value (PPV) of 19.3%, the reseauthorchers concluded that IRT could be recommended as a complementary method that requires other confirmatory diagnostic techniques.
The effects of pathologies and thermostability have been studied in other zoo species, such as equids, large felines, large ruminants, and wild birds, among others. Da Costa et al. [56] used IRT with 18 captive canines, including the crab-eating fox (Cerdocyon thous), maned wolf (Chrysocyon brachyurus), and hoary fox (Pseudalopex vetulus) in a study that lasted almost a year. Readings showed that the temperature of the left ear increased, compared to the right ear (24.6 and 21.3 °C, respectively). This effect was attributed to an abscess that was not perceivable in a remote inspection. In addition, it was also possible to verify post-treatment efficacy since the thermographic values were equal in both ears after 9 days of treatment. A study of giant sable antelopes (Hippotragus niger) and mishmi takins (Budorcas taxicolor taxicolor) used thermography to evaluate the effects of heat stress and how it influenced the animals’ behavior and thermoregulation mechanisms [1]. In contrast, in a study of a feline patient (Leopardus tigrinus) with renal failure, IRT did not show diagnostic validity or reveal any abnormal thermographic patterns in the animal’s pads due to the thickness of the dermis, which impeded taking accurate infrared reading [56]. Similarly, the ear region of Koalas, unlike the studies of elephants mentioned above, proved to be the least consistent window (compared to the ocular, abdominal, dorsal, and paws), as it showed a coefficient variation of only 19.68% and an average temperature of 22.11 °C, as opposed to the ocular IRT readings, which had a coefficient variation of 4.83% [26].
Finally, the application of IRT with captive wildlife species could be adapted for use with animals living in the wild. For example, auricular IRT has been evaluated in chimpanzees concerning behavioral changes, such as whimpers and aggressive barks, to assess the effect of emotions in response to conspecific vocalizations. In those test animals, after listening to aggressive bark vocalizations, the auricular surface temperature increased (p < 0.05) by an average of 0.7 ± 0.25 °C, but the temperature increase was greater during neutral calls due to an increase in blood flow that improved the performance of the animals’ auditory sensitivity to the vocalizations of their conspecifics [57]. These data demonstrate the usefulness of the auricular/pinna region for assessing various animal processes, especially the limits of thermoneutrality within which species held in captivity must be maintained to avoid behavioral and physio-metabolic complications triggered by heat stress.
References
- Hilsberg-Merz, S. Infrared Thermography in Zoo and Wild Animals. In Zoo and Wild Animal Medicine; Elsevier: Amsterdam, The Netherlands, 2008.
- Cannas, S.; Palestrini, C.; Canali, E.; Cozzi, B.; Ferri, N.; Heinzl, E.; Minero, M.; Chincarini, M.; Vignola, G.; Dalla Costa, E. Thermography as a Non-Invasive Measure of Stress and Fear of Humans in Sheep. Animals 2018, 8, 146.
- Leite, R.F.; Rodrigues, L.A.; Freitas, N.M.A.; Nogueira, F.R.B.; da Silva, L.J.; De Souza, B.B.; de Miranda Neto, E.G. Principles and Cautions in the Infrared Thermography Application in Sheep. Multidiscip. Rev. 2022, 5, e2022001.
- Elias, B.; Starling, M.; Wilson, B.; McGreevy, P. Influences on Infrared Thermography of the Canine Eye in Relation to the Stress and Arousal of Racing Greyhounds. Animals 2021, 11, 103.
- Dela Ricci, G.; Silva-Miranda, K.O.; Titto, C.G. Infrared Thermography as a Non-Invasive Method for the Evaluation of Heat Stress in Pigs Kept in Pens Free of Cages in the Maternity. Comput. Electron. Agric. 2019, 157, 403–409.
- Giannetto, C.; Di Pietro, S.; Falcone, A.; Pennisi, M.; Giudice, E.; Piccione, G.; Acri, G. Thermographic Ocular Temperature Correlated with Rectal Temperature in Cats. J. Therm. Biol. 2021, 102, 103104.
- Arfuso, F.; Rizzo, M.; Giannetto, C.; Giudice, E.; Fazio, F.; Piccione, G. Age-Related Changes of Serum Mitochondrial Uncoupling 1, Rumen and Rectal Temperature in Goats. J. Therm. Biol. 2016, 59, 47–51.
- Arfuso, F.; Acri, G.; Piccione, G.; Sansotta, C.; Fazio, F.; Giudice, E.; Giannetto, C. Eye Surface Infrared Thermography Usefulness as a Noninvasive Method of Measuring Stress Response in Sheep during Shearing: Correlations with Serum Cortisol and Rectal Temperature Values. Physiol. Behav. 2022, 250, 113781.
- Hilsberg-Merz, S. Infrared Thermography in Zoo Animals: New Experiences with This Method, Its Use in Pregnancy and Inflammation Diagnosis and Survey of Environmental Influences and Thermoregulation in Zoo Animals. Eur. Assoc. Zoo Wildl. Vet. 1998, 3, 397–409.
- Jerem, P.; Herborn, K.; McCafferty, D.; McKeegan, D.; Nager, R. Thermal Imaging to Study Stress Non-Invasively in Unrestrained Birds. J. Vis. Exp. 2015, 2015, 53184.
- Mota-Rojas, D.; Martínez-Burnes, J.; Casas-Alvarado, A.; Gómez-Prado, J.; Hernández-Ávalos, I.; Domínguez-Oliva, A.; Lezama-García, K.; Jacome-Romero, J.; Rodríguez-González, D.; Pereira, A.M.F. Clinical Usefulness of Infrared Thermography to Detect Sick Animals: Frequent and Current Cases. CABI Rev. 2022, 22, 1–27.
- Speakman, J.; Ward, S. Infrared Thermography: Principles and Applications. Zool. complex Syst. 1998, 101, 224–232.
- Church, J.; Cook, N.; Schaefer, A. Recent Applications of Infrared Thermography for Animal Welfare and Veterinary Research: Everything from Chicks to Elephants; InfraMation: Las Vegas, NV, USA, 2009.
- Whitham, J.; Miller, L. Using Technology to Monitor and Improve Zoo Animal Welfare. Anim. Welf. 2016, 25, 395–409.
- McCafferty, D.J. The Value of Infrared Thermography for Research on Mammals: Previous Applications and Future Directions. Mamm. Rev. 2007, 37, 207–223.
- Gómez-Prado, J.; Pereira, A.M.F.; Villanueva-García, D.; Wang, D.; Domínguez-Oliva, A.; Mora-Medina, P.; Hernández-Avalos, I.; Martínez-Burnes, J.; Casas-Alvarado, A.; Olmos-Hernández, A.; et al. Thermoregulation mechanisms and perspectives for validating thermal windows in pigs with hypothermia and hyperthermia: An overview. Front. Vet. Sci. 2022, 9, 1023294.
- Mota-Rojas, D.; Wang, D.; Titto, C.G.; Gómez-Prado, J.; Carvajal-de la Fuente, V.; Ghezzi, M.; Boscato-Funes, L.; Barrios-García, H.; Torres-Bernal, F.; Casas-Alvarado, A.; et al. Pathophysiology of Fever and Application of Infrared Thermography (IRT) in the Detection of Sick Domestic Animals: Recent Advances. Animals 2021, 11, 2316.
- Rekant, S.I.; Lyons, M.A.; Pacheco, J.M.; Arzt, J.; Rodriguez, L.L. Veterinary Applications of Infrared Thermography. Am. J. Vet. Res. 2016, 77, 98–107.
- Hilsberg-Merz, S. Aspekte Zur Klinischen Anwendung Der Infrarot-Thermographie in Der Zoo- Und Wildtiermedizin. Ph.D. Thesis, Universität Leipzig, Leipzig, Germany, 2000.
- Schneider, M. Behavioural and Autonomic Thermoregulation in Malayan Sun Bears (Helarctos Malayanus) and Polar Bears (Ursus Maritimus). Ph.D. Thesis, Universität zu Köln, Köln, Germany, 2015.
- Stryker, J.A. Thermoregulatory Behaviour Assessment and Thermal Imaging of Large Felids. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2016.
- Weissenböck, N.M.; Weiss, C.M.; Schwammer, H.M.; Kratochvil, H. Thermal Windows on the Body Surface of African Elephants (Loxodonta Africana) Studied by Infrared Thermography. J. Therm. Biol. 2010, 35, 182–188.
- Kuhn, R.; Meyer, W. Infrared Thermography of the Body Surface in the Eurasian Otter Lutra Lutra and the Giant Otter Pteronura Brasiliensis. Aquat. Biol. 2009, 6, 143–152.
- Okrouhlík, J.; Burda, H.; Kunc, P.; Knížková, I.; Šumbera, R. Surprisingly Low Risk of Overheating during Digging in Two Subterranean Rodents. Physiol. Behav. 2015, 138, 236–241.
- van Vuuren, A.K.; Kemp, L.V.; McKechnie, A.E. The Beak and Unfeathered Skin as Heat Radiators in the Southern Ground-hornbill. J. Avian Biol. 2020, 51, jav.02457.
- Narayan, E.; Perakis, A.; Meikle, W. Using Thermal Imaging to Monitor Body Temperature of Koalas (Phascolarctos Cinereus) in A Zoo Setting. Animals 2019, 9, 1094.
- Hilsberg-Merz, S. Clinical Application of Infrared-Thermography in Inflammation Diagnosis in Mega-Herbivores. In Proceedings of the European Association of Zoo- and Wildlife Veterinarians (EAZWV) 4th Scientific Meeting, Heidelberg, Germany, 8–12 May 2002; EAZWV: Heidelberg, Germany, 2002; pp. 315–320.
- Hurley-Sanders, J.L.; Bowman, K.F.; Wolfe, B.A.; Nutter, F.B.; Sladky, K.K.; Stoskopf, M.K. Use of Thermography and Fluorescein Angiography in the Management of a Chilean Flamingo with Avascular Necrosis of the Wing. J. Avian Med. Surg. 2012, 26, 255–257.
- Dunbar, M.R.; MacCarthy, K.A.; Dunbar, M.R.; MacCarthy, K.A. Use of Infrared Thermography to Detect Signs of Rabies Infection in Raccoons (Procyon Lotor). J. Zoo Wildl. Med. 2006, 37, 518–523.
- Dunbar, M.R.; Johnson, S.R.; Rhyan, J.C.; McCollum, M. Use of Infrared Thermography to Detect Thermographic Changes in Mule Deer (Odocoileus Hemionus) Experimentally Infected with Foot-and-Mouth Disease. J. Zoo Wildl. Med. 2009, 40, 296–301.
- Hurley-Sanders, J.L.; Sladky, K.K.; Nolan, E.C.; Loomis, M.R. Use of Cortical Bone Fenestration, Autogenous Free Skin Graft, and Thermography for Wound Treatment and Monitoring in a Red Wolf (Canis Rufus Gregoryi). J. Zoo Wildl. Med. 2015, 46, 617–620.
- Nakayama, K.; Goto, S.; Kuraoka, K.; Nakamura, K. Decrease in Nasal Temperature of Rhesus Monkeys (Macaca Mulatta) in Negative Emotional State. Physiol. Behav. 2005, 84, 783–790.
- Heintz, M.R.; Fuller, G.; Allard, S. Exploratory Investigation of Infrared Thermography for Measuring Gorilla Emotional Responses to Interactions with Familiar Humans. Animals 2019, 9, 604.
- Ross, S.R.; Lake, B.R.; Fultz, A.; Hopper, L.M. An Evaluation of Thermal Imaging as a Welfare Monitoring Tool for Captive Chimpanzees. Primates 2021, 62, 919–927.
- Stewart, M.; Webster, J.; Schaeder, A. Infrared Thermography and Heart Rate Variability for Non-Invasive Assessment of Animal Welfare. ANZCCART Hum. Sci. News 2008, 21, 1–6.
- Schaefer, A.L.; Cook, N.; Tessaro, S.V.; Deregt, D.; Desroches, G.; Dubeski, P.L.; Tong, A.K.W.; Godson, D.L. Early Detection and Prediction of Infection Using Infrared Thermography. Can. J. Anim. Sci. 2004, 84, 73–80.
- Lush, J.; Ijichi, C. A Preliminary Investigation into Personality and Pain in Dogs. J. Vet. Behav. 2018, 24, 62–68.
- Mota-Rojas, D.; Pereira, M.F.A.; Wang, D.; Martínez-Burnes, J.; Ghezzi, M.; Hernández-Ávalos, I.; Lendez, P.; Mora-Medina, P.; Casas, A.; Olmos-Hernández, A.; et al. Clinical Applications and Factors Involved in Validating Thermal Windows in Large Rumiants to Assess Health and Productivity. Animals 2021, 11, 2247.
- Butterfield, C.; Grumpelt, B.; Kimmel, D.; Patterson, R.; Jones, K.; Scott, S.L.; Schaefer, A. The Pretransport Management of Stress in Performance Horses. J. Equine Vet. Sci. 2018, 69, 145–148.
- Melero, M.; Rodríguez-Prieto, V.; Rubio-García, A.; García-Párraga, D.; Sánchez-Vizcaíno, J.M. Thermal Reference Points as an Index for Monitoring Body Temperature in Marine Mammals. BMC Res. Notes 2015, 8, 411.
- South, K.E. Infrared Thermography of Erinaceus Europaeus: Applications with Hypothermia and Hibernation. Ph.D. Thesis, University of Plymouth, Plymouth, UK, 2018.
- Pizzi, R.; Dowling, A.; Brown, D.; Girling, S.; Pearson, S.; Bacon, H.; Pereira, Y.M. Facial Thermography Is Not Useful in Assessing Body Temperature in Common Squirrel Monkeys (Saimiri Sciureus) in Comparison to Rectal Temperatures. J. Zoo Aquarium Res. 2015, 3, 94–98.
- Jansson, A.; Lindgren, G.; Velie, B.D.; Solé, M. An Investigation into Factors Influencing Basal Eye Temperature in the Domestic Horse (Equus Caballus) When Measured Using Infrared Thermography in Field Conditions. Physiol. Behav. 2021, 228, 113218.
- Heintz, M.R.; Fuller, G.A.; Allard, S.M. Welfare Assessment Using Infrared Thermography and Hormonal Measures During a Giraffe Feeding Program with Visitors. Available online: https://assets.speakcdn.com/assets/2332/heintz_giraffeirtaza2017poster.pdf (accessed on 12 September 2022).
- Vaz, J.; Bartley, A.; Hunt, J. Personality Matters: Exploring the Relationship between Personality and Stress Physiology in Captive African Lions. BMC Zoo. 2021, 7, 30.
- Ijichi, C.; Evans, L.; Woods, H.; Yarnell, K. The Right Angle: Validating a Standardised Protocol for the Use of Infra-Red Thermography of Eye Temperature as a Welfare Indicator. Anim. Welf. 2020, 29, 123–131.
- Bukhari, S.S.U.H.; McElligott, A.G.; Parkes, R.S.V. Quantifying the Impact of Mounted Load Carrying on Equids: A Review. Animals 2021, 11, 1333.
- Meleppat, R.K.; Fortenbach, C.R.; Jian, Y.; Martinez, E.S.; Wagner, K.; Modjtahedi, B.S.; Motta, M.J.; Ramamurthy, D.L.; Schwab, I.R.; Zawadzki, R.J. In Vivo Imaging of Retinal and Choroidal Morphology and Vascular Plexuses of Vertebrates Using Swept-Source Optical Coherence Tomography. Transl. Vis. Sci. Technol. 2022, 11, 11.
- Meleppat, R.K.; Zhang, P.; Ju, M.J.; Manna, S.K.; Jian, Y.; Pugh, E.N.; Zawadzki, R.J. Directional Optical Coherence Tomography Reveals Melanin Concentration-Dependent Scattering Properties of Retinal Pigment Epithelium. J. Biomed. Opt. 2019, 24, 1.
- Phillips, P.K.; Heath, J.E. Heat Exchange by the Pinna of the African Elephant (Loxodonta Africana). Comp. Biochem. Physiol. Part A Physiol. 1992, 101, 693–699.
- Isaza, R.; Hunter, R. Drug Delivery to Captive Asian Elephants—Treating Goliath. Curr. Drug Deliv. 2004, 1, 291–298.
- Rees, P.A. Asian Elephants (Elephas Maximus) Dust Bathe in Response to an Increase in Environmental Temperature. J. Therm. Biol. 2002, 27, 353–358.
- Koffi, M.; Andreopoulos, Y.; Jiji, L.M. The Role of Pinnae Flapping Motion on Elephant Metabolic Heat Dissipation. J. Heat Transfer 2014, 136, 101101.
- Marchant, G.H.; Shoshani, J. Head Muscles of Loxodonta Africana and Elephas Maximus with Comments on Mammuthus Primigenius Muscles. Quat. Int. 2007, 169–170, 186–191.
- Avni-Magen, N.; Zaken, S.; Kaufman, E.; Kelmer, G. Use of Infrared Thermography in Early Diagnosis of Pathologies in Asian Elephants (Elephas Maximus). Isr. J. Vet. Med. 2017, 72, 22–27.
- da Costa, A.L.M.; Rassy, F.B.; Cruz, J.B. da Diagnostic applications of infrared thermography in captive brazilian canids and felids. Arch. Vet. Sci. 2020, 25, 2.
- Dezecache, G.; Zuberbühler, K.; Davila-Ross, M.; Dahl, C.D. Skin Temperature Changes in Wild Chimpanzees upon Hearing Vocalizations of Conspecifics. R. Soc. Open Sci. 2016, 4, 160816.
More
