Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Sergio Terracina and Version 2 by Rita Xu.
Both physiological and pathological aging processes induce brain alterations especially affecting the speed of processing, working memory, conceptual reasoning and executive functions. As aging is partly contributed by free radical reactions, it has been proposed that exogenous antioxidants could have a positive impact on both aging and its associated manifestations.
- aging
- antioxidants
- cognition
- epigenetic
- hydroxytyrosol
- resveratrol
1. Introduction
It is well established that both the physiological and pathological aging processes induce brain alterations affecting, particularly, some aspects of cognition, especially the speed of processing, working memory, conceptual reasoning, and executive functions [1][2][3][1,2,3]. Even though the majority of the aged people retain relatively well-preserved health, this trend reflects on numerous individuals, especially those with disability and fragility [4][5][6][4,5,6]. However, there is significant heterogeneity among older adults in the rate of decline in some abilities, such as the measures of perceptual reasoning and processing speed [7]. More precisely, age-related brain deterioration results in a scaffolding of new compensatory networks, depending on the factors that positively and negatively influence cognition. This decline is mostly associated with a dysfunction of the pre-frontal cortex, which, being especially vulnerable, tends to atrophy prematurely while aging, causing a reorganization of brain functioning, which often occurs with hemispheric lateralization of the solicited regions with more frequent bilateral brain activation [8][9][10][8,9,10]. Furthermore, many pathologies, such as common comorbidities in elder people, negatively affect cognition, though further studies are required to better understand how aging plays a role and how brain structure and brain function might mediate the relationship between comorbidities and age on cognition [11][12][13][11,12,13].
2. Aging
2.1. General Mechanisms
Aging is an inevitable time-dependent decline of all biological functions, driven by a genetic program and linked to an increased risk for numerous diseases [14][16]. It has been suggested that the activation of multiple pathways due to the altered function of quality control systems monitoring the performance of the genomic and proteomic repertoire of the cells plays a major role [15][17]. In 2013, a total of nine biological hallmarks of aging have been identified: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, mitochondrial dysfunction, cellular senescence, deregulated nutrient sensing, stem cell exhaustion, and altered intercellular communication [16][17][18][18,19,20]. Actually, most chronic neurodegenerative human diseases (Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, etc.) are inherently associated with increasing age, as cells with senescence features have been detected in both brains of elders and patients with neurodegenerative disease, where they promote dysfunction [19][20][21,22]. In fact, senescent cells are characterized by sustained cell cycle arrest and production of a distinct senescence-associated secretory phenotype, and they accumulate with age and age-related diseases throughout the body, where they actively promote tissue decay. Human aging and neurodegenerative diseases comprise a series of changes at the molecular, cellular, physiological, and functional levels [21][23]. Interestingly, among the functional alterations, the cognitive, emotional, and social deficiencies are very common and are mostly linked to brain alterations. Furthermore, among the cellular changes, a major role is played by oxidative stress alterations. Since these alterations are mostly inevitable, it has been suggested that the main objectives of medical interventions for elders and by extension to neurodegenerative disease patients should focus on maximizing the ability of an individual to function in his environment, maintaining autonomy and maximizing quality of life [21][23].2.2. Brain Aging
Aging affects the brain and cognition because of multiple heterogeneous etiologies including alterations at various levels: molecular and cellular, vasculature, gross morphology, and cognition [22][24]. Numerous are the molecular and cellular changes brought by the aging process in the brain; these are characterized by a gradual reduction of neurophysiological functions, impaired adaptive neuroplasticity, dysregulated neuronal Ca2+ homeostasis, neuroinflammation, and oxidative alteration of molecules and organelles [23][25]. Brain aging-associated cellular and molecular changes are often related to neurodegenerative diseases due to increased oxidative stress, inflammation, energy metabolism disorders such as deregulated autophagy, mitochondrial dysfunction, and modifications of IGF-1, mTOR, ROS, AMPK, SIRTs, and p53 as central modulators of the metabolic control [24][26]. Interestingly, calorie restriction, physical exercise, and mental activities seem to be able to extend lifespan and increase nervous system resistance to age-associated neurodegenerative diseases, increasing protection against ROS generation, maintaining cellular Ca2+ homeostasis, and inhibiting apoptosis. Our vascular changes and our blood pressure tend to rise, increasing the risk of stroke and ischemia and causing white matter lesions of various sizes. Aging blood vessels are characterized by shrunk blood flow, potentially leading to organ atrophy and loss of function, that in the case of cerebral vascular aging can cause loss of the blood–brain barrier integrity, eventually resulting in cognitive and sensorimotor decline as well as diseases such as vascular cognitive impairment and dementia (VCID) due to chronic cerebral hypoperfusion [25][27] (see Figure 1).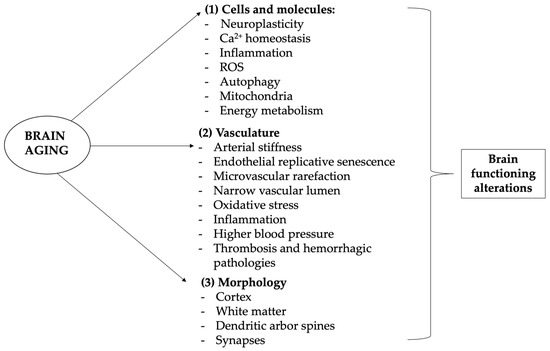
Figure 1. Brain-related aging mechanisms. Aging and neurodegenerative diseases are associated with cognitive, emotional, and social deficiencies mostly linked to brain alterations. Aging of the brain differs from other organs aging, as neurons are highly differentiated postmitotic cells, so that their lifespan is mostly equal to the lifespan of the entire organism. Brain aging is complex and heterogeneous but it substantially involves four levels of involvement: molecular and cellular, vasculature, gross morphology, and cognition. (1) Cellular and molecular changes involve especially (but not only) calcium-altered homeostasis, leading to hormone and neurotransmission changes, as well as ROS production, energy metabolism alteration, and neuroinflammation, which lead to progressive DNA and macromolecules damage, mitochondrial dysfunction, inflammation reaction, apoptosis, and epigenetic modifications; (2) vascular alterations and related disorders are very common and one of the leading causes of neurological disorders, morbidity, and mortality in older patients, manifesting its influence both systemically and on the more specific brain context; (3) with age come modifications of brain structure, with the frontal and pre-frontal lobes more influenced and occipital ones less affected; (4) cellular and molecular changes, but also vascular alterations and brain morphology modifications, are associated to functional impairment, which manifests mainly with memory loss and slight cognitive impairment but can lead to major pathological diseases such as dementia. In this context, antioxidants may play a major role in preventing cognitive aging problems.
3. Oxidative Stress
3.1. Oxidative Stress and Epigenetics
Oxidative stress or free radicals refer to an imbalance between reactive oxygen species (ROS) generation and antioxidant defense systems, which are implicated in different pathways of injury in the development of various disorders (including neurodegenerative disorders and aging) [14][33][34][35][16,35,36,37]. Interestingly, oxidative stress plays a major role in the aging process, both by direct damage and by causing epigenetic changes. Epigenetics is defined as a heritable regulation of gene expression through DNA and histone protein modifications without DNA sequence alteration [36][38]. Epigenetic modification is technically a reversible process, switching on/off genes in order to dynamically respond to the cellular milieu [37][39]. Dysregulation of epigenetics is frequently found in physiological conditions such as in aging but also in almost all diseases (especially cancers) [38][40]. Oxidative stress and epigenetic alterations usually coincide in diseases, suggesting a close relationship between these two events (Figure 2). It has been demonstrated that ROS cause global DNA hypomethylation, promoter hypermethylation, and altered histone modification, while epigenetic regulation of ROS-mediated processes suggests the possibility of promising tools to deepen in theour comprehension of the process of senescence, and to develop novel therapeutic strategies [39][40][41][41,42,43]. Among the highlighted tools to counter the harmful epigenetic effects of oxidative stress, dietary nutrients seem to be placed in a high spot [42][43][44][44,45,46].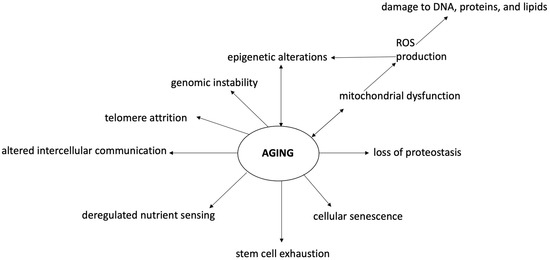
Figure 2. Oxidative stress and epigenetic changes in the cognitive aging process. Aging and neurodegenerative diseases are associated with cognitive, emotional, and social deficiencies mostly linked to brain alterations. Aging is characterized by a state of genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, mitochondrial dysfunction, cellular senescence, deregulated nutrient sensing, stem cell exhaustion, and altered intercellular communication. In this context, oxidative stress and epigenetic modifications play a major role, both as perpetrators and consequences of aging processes. Oxidative stress and ROS increase cause DNA and macromolecule damage associated with mitochondrial dysfunction, inflammation reaction, apoptosis, and epigenetic modifications. Epigenetic changes are associated with DNA hypomethylation, promoter hypermethylation, and altered histone modification due to various mechanisms (including oxidative stress). In this context, antioxidants may play a major role in preventing cognitive aging problems. ROS—reactive oxygen species.
3.2. Role of Mitochondria
Mitochondria are intracellular organelles, manned with their own circular genome (mtDNA), which play a major role in maintaining cellular homeostasis—by producing adenosine triphosphate (ATP) and intermediate metabolites—and regulating energy metabolism, cell survival and proliferation, and Ca2+ signaling [45][46][47,48]. Mitochondria are critical regulators of cell death, a key feature of neurodegeneration. As mutations in mitochondrial DNA and oxidative stress both contribute to aging, which is the greatest risk factor for neurodegenerative disease, the evidence suggests that mitochondria also have a central role in aging-related neurodegenerative diseases [47][49]. Despite being well-known hallmarks of aging, recent findings have revealed a novel crosstalk between histone epigenetic modifications and oxidative stress during stem cell aging, which once more highlights the importance of these issues for aging and age-related diseases [48][50]. Evidence supports the major role of mitochondrial dysfunction in promoting aging and in supporting neurodegenerative progression [49][51]. Mutations accumulate at a higher rate in mtDNA than in nuclear DNA, resulting in mitochondrial dysfunction and diseases, and this is even more true in older people, where there coexists a reduced mitochondrial efficiency and a deterioration of the antioxidant system [50][51][52][52,53,54]. Studies in various species highlighted several alterations in mitochondria and mitochondrial DNA (mtDNA) associated with aging: increased disorganization of mitochondrial structure; decline in mitochondrial oxidative phosphorylation function; accumulation of mtDNA mutation; increased mitochondrial production of ROS (superoxide, hydrogen peroxide, hydroxyl radicals and singlet oxygen); increased extent of oxidative damage to DNA, proteins, and lipids [53][55]. Thus, the decline in mitochondrial energy metabolism that alters quality control pathways, the enhanced mitochondrial oxidative stress, and the accumulation of mtDNA mutations are important contributors to human aging. Since the efficacy of the respiratory chain diminishes, aging is associated with electron leakage, increased ROS production and reduced cellular ATP generation [54][55][56,57]. Mitochondria have been related also to multiple diseases, often aging-related, such as neurodegeneration and cancer [56][57][58][58,59,60]. Interestingly, it has been suggested that novel pathways that protect the cell through mitochondrial quality control may offer unique opportunities for disease therapy in situations where ongoing mitochondrial damage occurs [59][61]. Some interesting studies demonstrated the major role of oxidative stress in regulating the lifespan, so reducing oxidative stress resulted in the expanded life in murine models [60][61][62,63]. In a major study, to determine the role of the reactive oxygen species in mammalian longevity and pathology, the researcheuthors generated transgenic mice that overexpress human catalase localized in the peroxisome, nucleus, or mitochondria [60][62]. In the mice overexpressing the human mitochondria catalase, the median and maximum lifespans were maximally increased (averages of 5 months and 5.5 months, respectively), while cardiac diseases and cataract development were delayed, oxidative damage was reduced, H2O2 production and H2O2− induced aconitase inactivation were attenuated, and the development of mitochondrial deletions was reduced. These results support the free radical theory of aging and reinforce the importance of mitochondria as a source of these radicals [60][61][62,63]. These results have been confirmed by another study that demonstrated how the overexpression of the antioxidant enzyme catalase in mitochondria can extend mouse lifespan, highlighting the importance of mitochondrial damage in aging and suggesting that, when targeted appropriately, boosting antioxidant defenses can increase mammalian life span [61][62][63,64]. Despite the need of further studies, therapies targeting basic mitochondrial processes, such as energy metabolism or free-radical generation, or specific interactions of disease-related proteins with mitochondria, hold great promise [47][49].3.3. Mediterranean Diet
The Mediterranean diet refers to a traditional diet consumed in Mediterranean countries and characterized by a high consumption of vegetables and olive oil and moderate consumption of food rich in proteins. It has been demonstrated that the Mediterranean diet, abundant in minimally processed plant foods, reduces the risk of developing various chronic diseases and seems to increase life expectancy [63][65]. In fact, this diet has beneficial effects in the primary and secondary prevention of cardiovascular disease, type 2 diabetes, atrial fibrillation, and breast cancer [64][65][66,67]. The exact mechanism by which an increased adherence to the Mediterranean diet exerts its favorable effects is mostly unknown; however, evidence suggests that the major role is played by: lipid-lowering effect, protection against oxidative stress, inflammation and platelet aggregation, modification of hormones and growth factors involved in the pathogenesis of cancer (reduction of DNA damages, cell proliferation, and their survival, angiogenesis, inflammations, and metastasis), inhibition of nutrient sensing pathways by specific amino acid restriction, and gut microbiota-mediated production of metabolites influencing metabolic health [64][66][67][68][66,68,69,70]. Interestingly, it has been demonstrated that the Mediterranean diet, and nutrients in general, can modulate gene expression directly by binding to nuclear receptors or acting indirectly modulating epigenetic effects (DNA methylation, histone modifications, microRNAs) [67][69][69,71]. Among the nutrients contained in the Mediterranean diet, olive oil and red wine are probably the most widely consumed and, when assumed at the correct dosage, they have been demonstrated to have beneficial effects on health [70][71][72,73]. In bringing these positive results, a major role is played by the presence of antioxidants in these aliments and, being among the most studied antioxidants, in the following chapters rwesearchers will report the experiences on hydroxytyrosol and resveratrol [72][73][74][74,75,76].3.4. Antioxidants
As antioxidants are substances that are efficient to trap ROS and decrease oxidative damage, these products have been studied for therapeutic approaches to many different diseases. On the other hand, a deficiency of antioxidants such as vitamins C and E has been associated with cognitive disorders [75][77]. In literature, many natural products and pharmacological compounds have antioxidant properties [71][76][73,78]. Interestingly, resveratrol and other polyphenols extracted from olive and wine but also other natural goods are among the most studied and possess great antioxidant and anti-inflammatory properties [77][78][79][80][79,80,81,82].3.5. Polyphenols
Polyphenols are natural, synthetic, or semi-synthetic organic molecules constituted by numerous hydroxyl groups on aromatic rings (phenolic groups), presenting neuroprotective and anti-inflammatory effects and capacity of control of oxidative stress, apoptosis and mitochondrial dysfunction [79][81][82][81,83,84]. These products are divided into four main groups: phenolic acids, flavonoids, stilbenes, and lignans. The Mediterranean diet is a mainstay of nutritional therapeutic and preventive programs in many diseases because of a rich presence of foods and beverages abundant in polyphenols, such as olives, olive oil, wine, fresh and processed fruits and vegetables, leguminous plants, cereals, herbs, spices, tea, coffee, and beer [83][84][85,86]. A proper diet is one of major factors contributing to good health and is directly related to the general condition of the organism [85][86][87,88]. Polyphenols are converted and absorbed mainly in the oral cavity and stomach; in the large intestine, the remaining polyphenols are further modified by bacterial enzymes (e.g., glycosides, esters, etc.) to obtain metabolites of lower-weight easier to absorb; these metabolites then circulate within blood, bound to proteins (mainly albumin), and are conjugated in the liver and kidneys; finally, elimination happens in the urine and feces [87][89]. Polyphenols are present in liquid natural products such as olive oil and green tea; however, it is also true that they can be found in alcohols such as red wine (whose main polyphenol is resveratrol) and beer [77][79][85][88][89][90][91][92][93][94][79,81,87,90,91,92,93,94,95,96]. Interestingly, the evidence suggests that moderate wine consumption may decrease the risk of several cancers (including colon, basal cell carcinoma, ovarian, and prostate cancer) and cognitive diseases; on the other hand, it should be pinpointed to an adequate balance in order to avoid the negative effects due to the presence of substances such as ethanol [95][96][97][97,98,99].3.6. Antioxidants and Cognition in the Aging Brain
Oxidative stress and the inflammation due to increased oxidative stress are associated to many chronic diseases, but the lack of anti-inflammatory drugs without side-effects has stimulated the search for new active substances. It has been demonstrated that the Central Nervous System (CNS) can benefit from nutritional strategies and dietary interventions that prevent the signs of senescence, such as cognitive decline or neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s Disease [98][100]. Both aging and associated neurodegenerative diseases are accompanied by the decline of several brain functions, including cognitive abilities, which are related to progressive deleterious changes at biochemical and physiological levels, leading to the generation of oxidative stress, disturbed protein metabolism with accumulation of protein aggregates, mitochondrial dysfunctions, loss of synaptic connections, and ultimately neurodegeneration and cognitive decline [99][101]. Because of its high energy demand, the brain is more susceptible to ROS-mediated damages, as it oxidizes lipids, proteins, and nucleic acids, thereby causing an imbalance in the homeostasis, and this especially occurs in the aging brain. It has been suggested that oxidative stress is a key factor for age-associated neurodegeneration and cognitive decline due to the imbalance between the rates of production and elimination of ROS. Interestingly, the involvement of the heme oxygenase (HO) pathway in anti-degenerative mechanisms related to the induction of other heat shock proteins (HSPs, molecular chaperones involved in cell protection from various forms of stress) has been demonstrated during various physio-pathological conditions [100][102]. In fact, the vasoactive molecule carbon monoxide and the potent antioxidant bilirubin, products of the HO-catalyzed reaction, represent a protective system that is potentially active against the brain oxidative injury associated to the cognitive dysfunction in the aging-associated neurodegenerative diseases [101][102][103,104]. Studies on both animal and human subject demonstrated that dietary interventions and plant-derived bioactive compounds with antioxidant properties could be beneficial for recovering the memory or delaying the onset of memory impairment, especially in case of stress-mediated changes [103][105]. Recently, the supplementation of spice and herbs containing phenolic substances with potent antioxidant and chemo-preventive properties, such as curcumin (a powerful antioxidant derived from the curry spice turmeric), has been considered as an alternative, nutritional approach to reduce oxidant damage and neurodegenerative pathology associated with aging. Recently, human studies have been conducted to determine the effective nutritional and lifestyle protocols for the prevention of neurodegenerative diseases [104][106]. The Mediterranean diet and antioxidant and anti-inflammatory products seem to play a significant role in most of the proposed protocols [105][107]. Furthermore, higher adherence to the Mediterranean dietary pattern has been associated with decreased cognitive decline and incidence of neurodegenerative diseases [106][108]. The example of two among the most studied antioxidant products and their role in preventing cognitive dysfunction due to brain aging: hydroxytyrosol and resveratrol (Figure 3 for a graphic description of the resveratrol and hydroxytyrosol molecular mechanisms of action).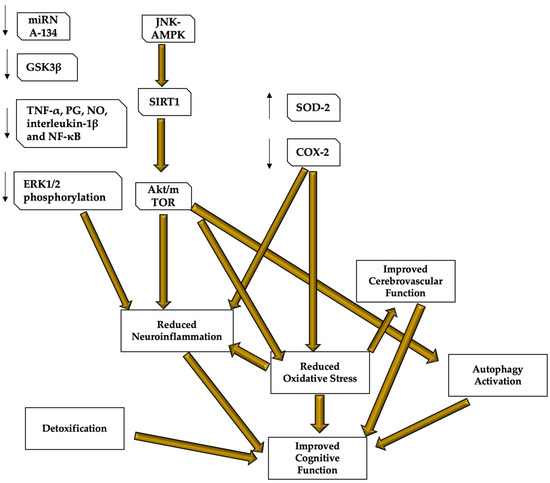
Figure 3. Resveratrol and hydroxytyrosol molecular mechanisms of action. Resveratrol and hydroxytyrosol demonstrated to be able to improve cognitive performance and protect the brain from neurodegenerative and age-related diseases, mostly thanks to their role in regulating oxidative stress, neuroinflammation, cerebral blood flow, and autophagy, as well as because of their capacity of detoxifying the blood from those neuro-damaging compounds. Most of the mechanisms are still under study, but it seems that both resveratrol and hydroxytyrosol target the AMPK and the subsequent pathway leading to SIRT1 and Akt/mTOR to reduce neuroinflammation and oxidative stress, and stimulating autophagy. Oxidative stress reduction also inhibits inflammatory responses and stimulates cerebrovascular function, leading to better cerebral blood flow and brain functioning. These compounds are also important detoxicants. Resveratrol mechanisms are clearer and its role in reducing neuroinflammation has been also related to the capacity of lowering mRNA134, GSK3β, ERK1/2 phosphorylation and cerebral levels of TNF-α, PG, NO, interleukin-1β and NF-κB. Resveratrol also increases SOD-2 protecting functions and inhibits COX-2 to reduce ROS production. AMPK—AMP-activated protein kinase; COX—cyclooxygenase; ERK—extracellular signal-regulated kinase; LPS—lipopolysaccharide; MMP9—matrix metalloproteinase 9; NF-κB—nuclear factor κB; NO—nitric oxide, PGES-1—prostaglandin E synthase-1; ROS—reactive oxygen species; TNF—tumor necrosis factor; SOD—superoxide dismutase.
